Allogeneic hematopoietic cell transplantation (HCT) after nonmyeloablative conditioning for hematologic malignancies depends on graft-versus-tumor effects for eradication of cancer. Here, we estimated relapse risks according to disease characteristics. Between 1997 and 2006, 834 consecutive patients (median age, 55 years; range, 5-74 years) received related (n = 498) or unrelated (n = 336) HCT after 2 Gy total body irradiation alone (n = 171) or combined with fludarabine (90 mg/m2; n = 663). Relapse rates per patient year (PY) at risk, corrected for follow-up and competing nonrelapse mortality, were calculated for 29 different diseases and stages. The overall relapse rate per PY was 0.36. Patients with chronic lymphocytic leukemia (CLL) and multiple myeloma (MM) in remission (CR), low-grade or mantle cell non-Hodgkin lymphoma (NHL) (CR + partial remission [PR]), and high-grade NHL-CR had the lowest rates (0.00-0.24; low risk). In contrast, patients with advanced myeloid and lymphoid malignancies had rates of more than 0.52 (high risk). Patients with lymphoproliferative diseases not in CR (except Hodgkin lymphoma and high-grade NHL) and myeloid malignancies in CR had rates of 0.26-0.37 (standard risk). In conclusion, patients with low-grade lymphoproliferative disorders experienced the lowest relapse rates, whereas patients with advanced myeloid and lymphoid malignancies had high relapse rates after nonmyeloablative HCT. The latter might benefit from cytoreductive treatment before HCT.
Introduction
We have developed a nonmyeloablative regimen for allogeneic hematopoietic cell transplantation (HCT) for the treatment of patients with hematologic malignancies.1,–3 The regimen has been translated from a preclinical canine model and uses conditioning with 2 Gy total body irradiation (TBI) with or without fludarabine, and postgrafting immunosuppression with an antimetabolite, mycophenolate mofetil (MMF), and a calcineurin inhibitor, cyclosporine (CSP).4 The latter 2 drugs are given for the dual purposes of enhancing engraftment and controlling graft-versus-host disease (GVHD). The regimen relies virtually entirely on graft-versus-tumor effects for eradicating cancer and can largely be administered in the ambulatory care setting because of the lack of serious regimen-related toxicities. The latter characteristic has enabled us to loosen the age and comorbidity limitations currently existing for myeloablative regimens.1,5,6 Given that and the fact that median ages at diagnosis for patients with most candidate diseases range from 65 to 70 years, the number of patients treatable by allogeneic HCT has been greatly increased. It is noteworthy that the regimen allows for the purest determination of graft-versus-tumor effects apart from conditioning and, therefore, provides an excellent foundation on which to add disease and disease stage–specific modalities (eg, targeted radiotherapy or cytoreductive autografts). The aim of the current retrospective analysis was to estimate relapse risks after HCT according to pretransplant disease characteristics and define groups of patients with low, standard, or high risks for relapse after HCT
Patients, materials, and methods
Eligibility criteria
Between December 17, 1997, and June 30, 2006, 834 patients with various hematologic malignancies were treated under different nonmyeloablative transplantation protocols within a consortium, consisting of the Fred Hutchinson Cancer Research Center (FHCRC), the University of Washington, Seattle Veterans Affairs Medical Center, Childrens Hospital and Regional Medical Center, all in Seattle, WA; Rocky Mountain Blood and Marrow Transplant Program, Denver, CO; Stanford University, Stanford, CA; the University of Leipzig, Leipzig, Germany; City of Hope National Medical Center, Duarte, CA; Baylor University, Dallas, TX; Oregon Health and Science University, Portland, OR; the University of Torino, Torino, Italy; Emory University, Atlanta, GA; University of Utah Health Sciences Center and LDS Hospital, Salt Lake City, UT; Medical College of Wisconsin, Milwaukee, WI; and the University Hospital Tübingen, Tübingen, Germany. FHCRC served as the coordinating center for all protocols. The Institutional Review Boards (IRB) at each of the collaborating centers approved the protocols. Patients signed consent forms that were in accordance with the Declaration of Helsinki and approved by the local IRBs. This retrospective analysis was approved by the IRB of the FHCRC.
Patients, conditioning regimen, and postgrafting immunosuppression
Patient and disease characteristics are shown in Table 1. A majority of the 834 patients had multiple myeloma (MM, n = 165), acute myeloid leukemia (AML, n = 152), or non-Hodgkin lymphoma (NHL, n = 146). The median patient age was 55 years (range, 5 to 74 years). There were 537 male patients and 297 female patients. Patients received either related (n = 498) or unrelated (n = 336) grafts. Patients and donors were matched for human leukocyte antigen (HLA) A, B, and C at least at the antigen level and for DRB1 and DQB1 at the allele level. Most patients received granulocyte colony stimulating factor-mobilized peripheral blood mononuclear cells (n = 816), and 18 received marrow (all unrelated grafts). Most patients were heavily pretreated, with a median of 3 preceding chemotherapy regimens (range 0-19), and a majority had measurable disease at time of transplant including patients with partial remission (PR), stable disease (SD), refractory disease, or untreated disease. One hundred seventy-six patients had failed high-dose autologous HCT, whereas 148 had planned preceding autologous HCT, 121 of whom were patients with MM.
Characteristics of the 834 patients undergoing nonmyeloablative conditioning
| Parameter . | Value . |
|---|---|
| Median age, y (range) | 55 (5-74) |
| Sex, n | |
| Male | 537 |
| Female | 297 |
| Hematopoietic graft, n | |
| G-PBMC | 816 |
| Marrow | 18 |
| Diagnosis, n | |
| Multiple myeloma | 165 |
| AML | 152 |
| NHL | 146 |
| MDS | 103 |
| CLL | 82 |
| Hodgkin lymphoma | 51 |
| CML | 47 |
| ALL | 30 |
| Myeloproliferative disease | 19 |
| Renal-cell carcinoma | 18 |
| CMML | 12 |
| Waldenström disease | 9 |
| No. of preceding chemotherapy regimens, median (range) | 3 (0-19) |
| Preceding autologous HCT, no. patients | |
| No | 510 |
| Yes* | 324 |
| Conditioning regimens, n | |
| 2 Gy TBI | 171 |
| 2 Gy TBI + fludarabine | 663 |
| Donor, n | |
| Related | 498 |
| Unrelated | 336 |
| Median duration of follow-up†, mo (range) | 37.1 (2.8-98.7) |
| Parameter . | Value . |
|---|---|
| Median age, y (range) | 55 (5-74) |
| Sex, n | |
| Male | 537 |
| Female | 297 |
| Hematopoietic graft, n | |
| G-PBMC | 816 |
| Marrow | 18 |
| Diagnosis, n | |
| Multiple myeloma | 165 |
| AML | 152 |
| NHL | 146 |
| MDS | 103 |
| CLL | 82 |
| Hodgkin lymphoma | 51 |
| CML | 47 |
| ALL | 30 |
| Myeloproliferative disease | 19 |
| Renal-cell carcinoma | 18 |
| CMML | 12 |
| Waldenström disease | 9 |
| No. of preceding chemotherapy regimens, median (range) | 3 (0-19) |
| Preceding autologous HCT, no. patients | |
| No | 510 |
| Yes* | 324 |
| Conditioning regimens, n | |
| 2 Gy TBI | 171 |
| 2 Gy TBI + fludarabine | 663 |
| Donor, n | |
| Related | 498 |
| Unrelated | 336 |
| Median duration of follow-up†, mo (range) | 37.1 (2.8-98.7) |
TBI indicates total body irradiation; AML, acute myeloid leukemia; NHL, non-Hodgkin lymphoma; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndromes; CML, chronic myeloid leukemia; ALL, acute lymphoblastic leukemia; CMML, chronic myelomonocytic leukemia; CR, complete remission; PR, partial remission; HCT, hematopoietic cell transplantation; G-PBMC, granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells.
Planned, n = 148; failed, n = 176; 121 patients with MM had planned autologous HCT (8 of these had failed another preceding autologous HCT) and an additional 29 had failed autologous HCT.
Follow-up duration for 390 surviving patients.
The conditioning regimen consisted of 2 Gy TBI on day 0 (dose rate 7 cGy/min from linear accelerators) given either alone or combined with fludarabine (30 mg/m2/day) on days −4, −3, −2 before HCT.1,–3 Immunosuppressive therapy with 5.0-6.25 mg CSP/kg, given orally twice a day, was started on day −3 (usually extended to day 180), and 15 mg MMF/kg, given orally 2 or 3 times a day, was started 4 to 6 hours after HCT and either extended to day 28 (related grafts) or day 96 (unrelated grafts). Intravenous formulations of CSP and MMF were administered if patients were unable to tolerate oral medications.
Relapse/progression
Relapse was defined as recurrence of malignancy based on one or more of the following parameters: marrow morphology, flow cytometry, cytogenetic studies, including fluorescence in situ hybridization, electrophoresis, immunofixation assays, polymerase chain reaction-based assays for disease markers, or imaging results.
Disease progression was defined as an increase of at least 50% in disease burden. Donor lymphocyte infusion or other therapeutic interventions were administered only after relapse/progression had been diagnosed.
Statistical analysis
To quantify the underlying rates of relapse according to diseases and disease stages, we calculated the relapse rate per patient year (PY) during the first 2 years after transplantation. For each disease group, the relapse rate per PY was the total number of observed relapse/progression events, divided by the total duration of follow-up. For each patient, the contributions to follow-up were the times from transplantation to the first occurrence of relapse/progression, death without relapse/progression, last contact, or 2 years. We used this quantity, instead of cumulative incidence at 2 years, because the latter reflected also the effect of nonrelapse mortality, in addition to the intrinsic rates of relapse among patients at risk. Survival curves were estimated using the Kaplan-Meier method. Data were analyzed as of February 1, 2007.
Results
Overall, 312 of the 834 patients experienced relapse/progression of their disease within the first 2 years after transplantation. The overall relapse per PY was 0.36. The relapse rate per PY was 0.35 among patients with related donors and 0.37 among those with unrelated donors. Seventeen of the 312 relapses occurred after graft rejection. A third of these rejections (n = 5) occurred among patients with CML in first chronic phase, whereas the remainder were scattered among different patient groups. An additional 36 patients experienced relapse/progression more than 2 years after transplant; however, these events were not used in the calculation of the relapse rates per PY. Excluding MM patients who had planned autologous HCT (see Table 1), there was no evidence that a history of failed autologous HCT was associated with a higher risk of relapse (HR = 1.06; P = .74).
Diseases with relapse rates per PY between 0.00 and 0.24 were grouped in the low-risk group (24% of all patients); those with relapse rates between 0.26 and 0.37 were considered standard risk (47% of all patients), and those with rates per PY higher than 0.52 were designated as high risk (29% of all patients) (Table 2).
Relapse rates in 29 diagnosis and disease stage groups
| Disease . | Disease stage . | Patients,N . | PY offollow-up* . | Relapse rateper PY . |
|---|---|---|---|---|
| Low risk | ||||
| CLL | CR | 7 | 8.2 | 0.00 |
| NHL, low grade | Not in CR | 34 | 40.8 | 0.15 |
| NHL, low grade | CR | 9 | 11.1 | 0.18 |
| Waldenström | Advanced | 9 | 10.8 | 0.19 |
| MM | CR† | 29 | 42.8 | 0.19 |
| NHL, mantle cell | CR | 16 | 15.7 | 0.19 |
| NHL, mantle cell | Not in CR | 25 | 30.4 | 0.20 |
| MPD | Advanced | 19 | 18.6 | 0.21 |
| NHL, high grade | CR | 26 | 31.0 | 0.23 |
| ALL | 1st CR | 19 | 21.0 | 0.24 |
| Standard risk | ||||
| CLL | Not in CR | 75 | 89.0 | 0.26 |
| MM | Not in CR | 136 | 174.8 | 0.27 |
| MDS | RA/RARS | 20 | 18.2 | 0.33 |
| AML | 1st CR | 80 | 78.3 | 0.33 |
| CML | 1st CP | 26 | 32.8 | 0.34 |
| AML | ≥2nd CR | 59 | 62.6 | 0.37 |
| High risk | ||||
| MDS | RAEB/RAEB-t | 23 | 19.2 | 0.52 |
| AML | Evolved from MDS | 42 | 29.1 | 0.55 |
| NHL, high grade | Not in CR | 36 | 26.3 | 0.57 |
| HD | CR | 13 | 9.6 | 0.62 |
| MDS | Secondary | 18 | 14.4 | 0.70 |
| HD | Not in CR | 38 | 30.6 | 0.72 |
| AML | Not in CR | 13 | 9.2 | 0.87 |
| CML | AP/BC | 14 | 10.1 | 0.99 |
| CML | 2nd CP | 7 | 3.8 | 1.05 |
| Renal cell | Metastatic | 18 | 10.6 | 1.23 |
| ALL | ≥2nd CR | 8 | 4.6 | 1.29 |
| ALL | Not in CR | 3 | 2.2 | 1.35 |
| CMML | Advanced | 12 | 6.3 | 1.42 |
| Disease . | Disease stage . | Patients,N . | PY offollow-up* . | Relapse rateper PY . |
|---|---|---|---|---|
| Low risk | ||||
| CLL | CR | 7 | 8.2 | 0.00 |
| NHL, low grade | Not in CR | 34 | 40.8 | 0.15 |
| NHL, low grade | CR | 9 | 11.1 | 0.18 |
| Waldenström | Advanced | 9 | 10.8 | 0.19 |
| MM | CR† | 29 | 42.8 | 0.19 |
| NHL, mantle cell | CR | 16 | 15.7 | 0.19 |
| NHL, mantle cell | Not in CR | 25 | 30.4 | 0.20 |
| MPD | Advanced | 19 | 18.6 | 0.21 |
| NHL, high grade | CR | 26 | 31.0 | 0.23 |
| ALL | 1st CR | 19 | 21.0 | 0.24 |
| Standard risk | ||||
| CLL | Not in CR | 75 | 89.0 | 0.26 |
| MM | Not in CR | 136 | 174.8 | 0.27 |
| MDS | RA/RARS | 20 | 18.2 | 0.33 |
| AML | 1st CR | 80 | 78.3 | 0.33 |
| CML | 1st CP | 26 | 32.8 | 0.34 |
| AML | ≥2nd CR | 59 | 62.6 | 0.37 |
| High risk | ||||
| MDS | RAEB/RAEB-t | 23 | 19.2 | 0.52 |
| AML | Evolved from MDS | 42 | 29.1 | 0.55 |
| NHL, high grade | Not in CR | 36 | 26.3 | 0.57 |
| HD | CR | 13 | 9.6 | 0.62 |
| MDS | Secondary | 18 | 14.4 | 0.70 |
| HD | Not in CR | 38 | 30.6 | 0.72 |
| AML | Not in CR | 13 | 9.2 | 0.87 |
| CML | AP/BC | 14 | 10.1 | 0.99 |
| CML | 2nd CP | 7 | 3.8 | 1.05 |
| Renal cell | Metastatic | 18 | 10.6 | 1.23 |
| ALL | ≥2nd CR | 8 | 4.6 | 1.29 |
| ALL | Not in CR | 3 | 2.2 | 1.35 |
| CMML | Advanced | 12 | 6.3 | 1.42 |
CLL indicates chronic lymphocytic leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; CR, complete remission; MPD, myeloproliferative disease; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CP, chronic phase; RAEB, refractory anemia with excess blasts; RAEB-t, refractory anemia with excess blasts in transformation; HD, Hodgkin disease; AP, accelerated phase; BC, blast crisis; CMML, chronic myelomonocytic leukemia.
PY of follow-up is the total person-years of observation time from transplant until death, relapse/progression, last contact, or 2 years.
The criteria for complete remission from MM were absence of monoclonal immunoglobulin and of discernible light chains in urine by standard electrophoresis, the absence of visible monoclonal bands on immunofixation, <1% plasma cells in marrow aspirates, the absence of evidence of clonal disease according to flow cytometry of marrow cells, and the absence of an increase in the size or number of osteolytic lesions.
Low-risk diseases included chronic lymphocytic leukemia (CLL) in CR (relapse rate per PY, 0.00); low-grade NHL, whether in CR or not (relapse rates per PY, 0.18 and 0.15, respectively); Waldenström disease (relapse rate per PY, 0.19); MM in CR (relapse rate per PY, 0.19); mantle cell NHL, whether in CR or not (relapse rates per PY, 0.19 and 0.20, respectively); myeloproliferative disease (MPD, relapse rate per PY, 0.21); high-grade NHL in CR (relapse rate per PY, 0.23); and acute lymphoblastic leukemia (ALL) in CR1 (relapse rate per PY 0.24).
The standard-risk group included patients with CLL or MM with measurable disease at HCT (relapse rates per PY, 0.26 and 0.27, respectively; 94 of the 136 patients with MM had preceding autologous HCT after high-dose melphalan7 ); myelodysplastic syndromes (MDS)-refractory anemia (RA)/refractory anemia ringed sideroblasts (RARS), AML in first CR, CML in first chronic phase (CP), and AML in second or later CR (relapse rates per PY ranging from 0.33 to 0.37).
The high-risk group included patients with myelodysplastic syndrome (MDS)-refractory anemia with excess of blasts (RAEB)/RAEB in transformation (RAEB-t), AML after MDS or AML not in CR, high-grade NHL not in CR, Hodgkin lymphoma, MDS after chemotherapy, CML in second CP or accelerated phase/blast crisis, ALL in second or later CR, chronic myelomonocytic leukemia (CMML), and renal cell carcinoma, with relapse rates per PY ranging from 0.52 to 1.42.
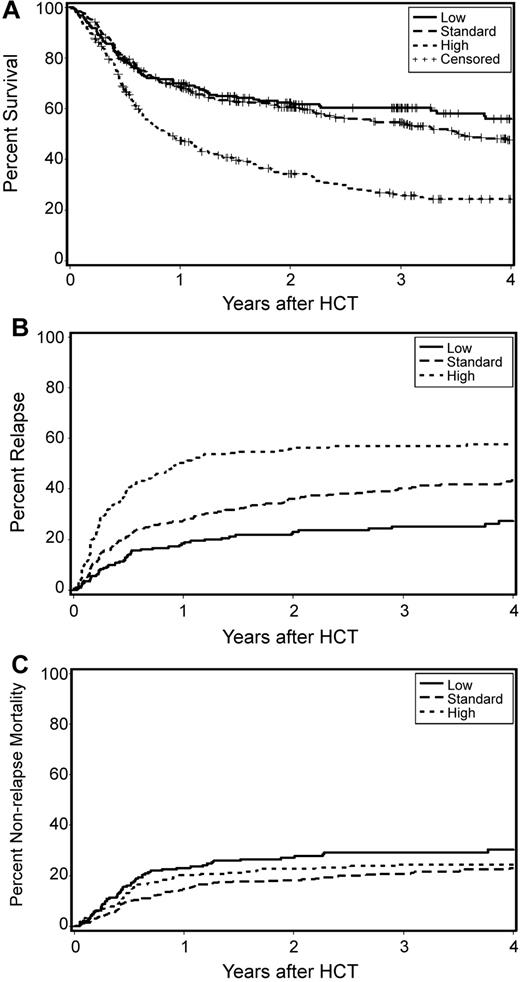
Figure 1 illustrates the impact of relapse risk on overall survival. Three-year survival for low-risk patients was 60% compared with 55% for those with standard risk and 26% for those in the high-risk group (Figure 1A). The survival differences were largely due to differences in relapse mortality (Figure 1B). The cumulative relapse incidences at 3 years were 25% for low-risk, 40% for standard-risk, and 57% for high-risk patients. In contrast, no significant differences in nonrelapse mortalities within the 3 risk groups were seen (Figure 1C). The cumulative nonrelapse mortality incidences at 3 years were 29% for low-risk, 21% for standard-risk, and 26% for high-risk patients.
Overall survivals (A), cumulative relapse rates (B), and cumulative nonrelapse mortality rates according to risk groups (C).
Overall survivals (A), cumulative relapse rates (B), and cumulative nonrelapse mortality rates according to risk groups (C).
Discussion
The success of allogeneic HCT in curing patients with hematologic malignancies depends, in part, on cytotoxic antitumor effects of conditioning regimens and, in part, on immune-mediated destruction of cancer cells through grafted cells. Target antigens for T-cell–mediated graft-versus-tumor effects can be ubiquitous polymorphic minor histocompatibility antigens and/or antigens uniquely expressed on hematopoietic cells (hematopoietic antigens),8,9 although a role for natural killer cells has also been postulated.10 The current conditioning regimen of 2 Gy TBI with or without fludarabine was designed to reduce toxicities and allow extending HCT to older and sicker patients and, therefore, has few cytotoxic antitumor effects. As a result, tumor eradication relies virtually entirely on graft-versus-tumor activities. These, in turn, can vary in intensity, depending on the immunogenicity of the tumors and the respective proliferation rates both of the tumors and the donor immune cells poised to destroy them.
The current study analyzed outcomes in 834 consecutive patients with various hematologic malignancies given either related or unrelated HCT and sought to determine which diseases and disease stages responded well and which less well to graft-versus-tumor effects as assessed by relapse rates per PY. Using this criterion, patients could be roughly divided into 3 groups that had either low, standard, or high risks of relapse, although nonrelapse mortalities among the 3 groups were comparable, the latter ranging from 21% to 29% at 3 years. Accordingly, differences in survival among the 3 groups were largely determined by differences in relapse rates. These ranged from as low as 0-0.24 relapses per PY in the low-risk group to 0.52-1.42 in the high-risk group, with the standard-risk patients placed in between (0.26-0.37). Three-year survival rates were 60% for the low-risk group, 55% for the standard-risk group, and 26% for the high-risk group.
The low-risk group included patients with lymphoid malignancies who were in CR (eg, CLL, ALL in CR1, Waldenström's, MM, and high-grade, mantle cell, and low-grade NHL [the latter 2 also included patients in PR]). The low tumor burden and slow-growing nature of these cancers combined with their presumed ability to present target antigens to the donor T cells were likely reasons for the low relapse rates and good posttransplantation survival. Similarly good outcomes in these diseases and disease stages have also been reported by others, with progression-free and overall survival rates ranging from 29.4% to 84% and 41% to 84%, respectively, although, as a rule, the conditioning regimens used were more intense. For example, Schetelig et al11 used a combination of fludarabine, busulfan, and antithymocyte globulin for conditioning. Dreger et al12 reported on various regimens, including fludarabine/cyclophosphamide and low-dose TBI; Khouri et al13 combined fludarabine with cyclophosphamide; Lee et al14 conditioned either with 100 mg melphalan/m2 (related donors) or melphalan/250 cGy TBI/fludarabine (unrelated donors); and Gerull et al15 used fludarabine/low-dose TBI, similar to the current study. Patient ages in these studies were slightly, but not significantly, lower than current patients. Donors were more often related than unrelated. Current results suggested that a minimal conditioning regimen that enabled sustained allogeneic engraftment might be sufficient in patients with low-grade B-cell malignancies in CR or PR.
The standard-risk group included patients with MM and CLL not in CR, and various early-stage myeloid malignancies. Although the balance was still in favor of the grafted donor immune cells, with the result that a majority of patients either achieved CR or remained in CR, it was clear that larger tumor burden, relatively faster proliferation rates of tumor cells, and perhaps variable sensitivity of cancer cells to donor immune cells adversely affected outcomes in a strong minority of patients who experienced relapse/progression. To improve outcomes and avoid across-the-board increases in conditioning intensity (and toxicity), individual patients at risk of relapse need to be identified. For example, in the case of patients with CML in CP1, relapse/recurrence was a direct consequence of an initially observed high rate of nonfatal graft rejections, and this problem has since been addressed by adding fludarabine to 2 Gy TBI or increasing the TBI dose to 3 Gy. In patients with CLL who had bulky lymphadenopathy and thus were at high risk of relapse, a single dose of a radiolabeled monoclonal antibody to CD20 has been added to the conditioning regimen.16 For other disease groups and stages, pinpointing risk factors might require larger numbers of patients, although the fact that almost half of the patients with AML in CR1 and CR2 were older than 60 years might have contributed to a higher relapse risk. Others have used a more intensive conditioning regimen of 8 Gy TBI plus fludarabine, and reported 60%-70% relapse-free survivals in a slightly younger cohort of patients with AML in first or second remission, supporting the notion that more intensive conditioning might be beneficial in some patients.17 Nevertheless, their reported survival of patients with more advanced AML was not better than that observed among a similar group of patients reported here. Data comparable with ours were described by Gupta et al,18 using fludarabine and 2 Gy TBI, and Sayer et al,19 using fludarabine and reduced doses of busulfan both in patients with AML who were in morphologic CR at HCT and those with more advanced disease. Khouri et al20 observed 83% 2-year survival in patients with CML in chronic phase conditioned with 550 cGy TBI (delivered at 35 cGy/min), a result that was comparable with the survival of those current patients who were conditioned with fludarabine and 2 Gy TBI.
The high-risk group was comprised of patients with advanced stages of NHL (not in CR), Hodgkin lymphoma, MDS, CML, CMML, acute leukemias, and renal cell carcinoma. Here, the large numbers of cancer cells present at HCT might have shifted the balance in their favor, and they “outproliferated” the cytotoxic donor immune cells in a majority of patients. Cytotoxic donor immune cells tended to work slowly, and even under the best of circumstances, say, in patients with slow growing B-cell malignancies such as CLL, graft-versus-tumor effects might take many months before accomplishing molecular remissions.21 It remains to be seen whether adding targeted therapy to the current regimen, including radiolabeled monoclonal antibodies to CD2016 or CD4522 expressed on tumor cells, will reduce the tumor burden sufficiently to tilt the balance toward the donor immune cells. Alternatively, for patients with MDS, a dose escalation of TBI to 3 Gy is being explored, in part to decrease the relatively high rate of graft failure. A strong hint that increasing the intensity of the conditioning regimen might improve outcomes comes from a study in patients with Hodgkin lymphoma; in that study, patients were conditioned with fludarabine, melphalan, and alemtuzumab, and had 4-year overall and progression-free survivals of 55.7% and 39%, respectively.23
In conclusion, allogeneic graft-versus-tumor effects are powerful and can lead to cures of otherwise incurable hematologic malignancies. They work best in patients with relatively low tumor burdens and slow growing tumors (eg, NHL or CLL) and least well in patients who have bulky tumors with relatively fast proliferation rates (eg, acute leukemias in relapse). To improve outcomes in such patients, either the option of allografting should be considered earlier in the disease course when the tumor burden is lower, or targeted therapies with limited systemic toxicities should be added, such as radiolabeled monoclonal antibodies directed against surface antigens that are specific for the tumor cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to the patients and their donors who participated in this study. In addition, we thank all physicians, nurses, and support personnel for their very dedicated care of patients on this study, the research nurses John Sedgwick, Mary Hinds, Michelle Bouvier, and data coordinator Heather Hildebrandt for their help in data acquisition, the long-term follow-up team, and Helen Crawford and Bonnie Larson for manuscript preparation.
This work was supported by National Institutes of Health grants CA78902, CA18029, CA15704, CA49605, CA30206, DK064715, and HL36444. C.K. was supported by a fellowship from Deutsche Krebshilfe, Dr Mildred-Scheel-Stiftung für Krebsforschung. B.B. received support from the Fondazione Cassa di Risparmio di Torino and Comitato Regionale Piemontese Gigi Ghirotti, Progetto Vita Vitae.
National Institutes of Health
Authorship
Contribution: C.K. performed retrospective data collection and drafted the manuscript. B.E.S. designed research, performed statistical analyses. B.M.S. and D.G.M. designed research protocol, entered patients on study, verified data, and participated in the writing of the manuscript. M.M. verified data and assisted in drafting the manuscript. M.B.M., K.G.B., D.N., T.R.C., S.J.F., E.A., J.F.L., B.B., A.L., P.A.M., J.C.W., E.E., F.B.P., and W.A.B. contributed patients to studies, verified patient data, and reviewed the manuscript. R.S. designed, directed, and provided funding for the study and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rainer Storb, MD, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave. N., D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail:rstorb@fhcrc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal