Factors regulating which patients become alloimmunized to red blood cell (RBC) antigens are poorly understood. Using a murine model of transfusion, we recently reported that viral-like inflammation with polyinosinic polycytidylic acid [poly (I:C)] significantly enhances RBC alloimmunization. Herein, we tested the hypothesis that poly (I:C) exerts this effect, at least in part, at the level of antigen-presenting cells (APCs). Using a novel in vivo method, we report that in the noninflamed state, most transfused RBCs were consumed by splenic macrophages, with only trace consumption by splenic dendritic cells (DCs). To a lesser extent, RBCs were also consumed by APCs in the liver. However, unlike soluble antigens, no RBCs were consumed by APCs in the lymph nodes. Inflammation with poly (I:C) induced significant consumption of transfused RBCs by splenic DCs, with a concomitant increase in costimulatory molecule expression. Moreover, this resulted in increased proliferation of CD4+ T cells specific for the mHEL RBC alloantigen. Finally, splenectomy abrogated the enhancing effects of poly (I:C) on RBC alloimmunization. Together, these data provide additional insight into the nature of transfused RBCs as an immunogen and provide a mechanism by which viral-like inflammation enhances alloimmunization to transfused RBCs.
Introduction
Transfusion of foreign red blood cells (RBCs) can result in production of antibodies against alloantigens on the transfused RBCs (RBC alloimmunization). Indeed, chronically transfused patients can become broadly alloimmunized against multiple RBC antigens, leading to a situation where insufficient numbers of compatible RBC units are available to support the patient's therapeutic needs. This results in significant morbidity, and in some cases, mortality. Despite the fact that every nonautologous unit of RBCs has multiple mismatched antigens as compared with those of the recipient, RBC alloimmunization is a fairly rare event. Depending on the study and detection methods, alloimmunization has been reported to occur in only 3% to 10% of multiply transfused patients,1,,,,–6 with higher rates reported in patients with hemoglobinopathies.7,,,–11 However, it remains unclear why some patients, but not others, become alloimmunized to RBC antigens
Genetic factors likely play a role in RBC alloimmunization, as the correct HLA-type is required to respond to some blood group antigens.12,13 However, having the correct HLA type is not alone sufficient to lead to alloimmunization. Moreover, responses to certain blood group antigens are not restricted to HLA type.13,14 Thus, in addition to HLA genetics, environmental factors may play a role in RBC alloimmunization. In support of this notion, rates of alloimmunization have been correlated to the underlying pathophysiology of the transfusion recipient,7,11,15,,,,–20 suggesting regulation of RBC alloimmunization by epigenetic host-specific factors.
It is generally understood that introduction of foreign antigen is weakly immunogenic in the absence of an adjuvant.21,22 In recent years, it has been appreciated that the immune-enhancing components of known adjuvants activate pathways of the innate immune system that have evolved to detect the presence of microbial infection. In many cases, whether or not an immune response occurs can be predicted by the presence or absence of activation of such pathways.23,24 This may help explain the relatively low rates of RBC alloimmunization, as a properly processed and transfused unit of RBCs should constitute a sterile antigen. In contrast, occurrence of alloimmunization may be regulated in part by introduction of an inflammatory stimulus, either through a contaminated unit of RBCs or from recipient pathology.
Consistent with this hypothesis, using a murine model of RBC alloimmunization, we have recently reported that administration of polyinosinic polycytidylic acid [poly (I:C)], a toll-like receptor 3 agonist that induces viral-like inflammation,25 significantly enhances both the frequency and magnitude of RBC alloimmunization.26 There are numerous points in the process of RBC alloimmunization by which poly (I:C)-induced inflammation may exert its effects. Herein, we test the hypothesis that poly (I:C) increases RBC alloimmunization by enhancing the processing and presentation of RBC alloantigens by recipient antigen-presenting cells (APCs).
We report that in the noninflamed state, transfused RBCs were consumed predominantly by macrophages in the spleen, with only minimal consumption by splenic dendritic cells (DCs). To a lesser extent, transfused RBCs were also consumed by macrophages and DCs in the liver. However, in contrast to many other antigens, transfused RBCs were not consumed by APCs in the lymph nodes. Analysis of poly (I:C) effects on splenic APCs revealed a significant increase of RBC consumption by splenic DCs, with a concomitant induction of costimulatory molecule expression. Furthermore, transfusion of mismatched RBCs containing the mHEL (membrane-bound hen egg lysosyme) antigen, in the presence of poly (I:C), led to subsequent enhancement of the proliferation of CD4+ T cells specific for the RBC alloantigen. Splenectomy abrogated the enhancing effects of poly (I:C) on RBC alloimmunization, suggesting that poly (I:C) enhances alloimmunization by affecting splenic tissues. Together, these data provide insight into the nature of RBCs as immunogens and provide mechanistic details of the effects of poly (I:C) on RBC alloimmunization at the level of APCs.
Materials and methods
Mice
C57BL/6 and B10.BR mice were purchased from the Jackson Laboratories (Bar Harbor, ME). mHEL mice, C57BL/6 × B10.BR mice, and 3A9 × B6.PL-Thy1.1 mice were bred by the Emory University Department of Animal Resources. Mice were maintained on standard rodent chow and water in a temperature- and light-controlled environment. Mice were used at 8 to 12 weeks of age, and all procedures were performed according to approved Institutional Animal Care and Use Committee (IACUC) protocols.
C57BL/6 mice were used as a general strain to study RBC consumption, and B10.BR mice were used for studies involving mHEL RBCs, as B10.BR are better responders to mHEL then are C57BL/6. The 3A9 mouse expresses a transgenic T-cell receptor that recognizes HEL peptide presented by major histocompatibility complex (MHC) class II,27 and is thus a tool to study anti-HEL CD4+ T-cell responses. The B6.PL-Thy1.1 mice express a congenic marker on T cells and were thus crossed with 3A9 mice to allow in vivo tracking and analysis of adoptively transferred 3A9 T cells.
Leukoreduction of murine blood
Blood from donor mice was obtained by retro-orbital bleeding, washed, and leukoreduced over a neonatal leukoreduction filter (Purecell; Pall Biomedical Products, East Hills, NY) as previously described.26
DiO labeling of murine blood
After leukoreduction, murine blood was washed and labeled with 3,3′-dihexadecyloxacarbocyanine perchlorate (DiO; Molecular Probes, Invitrogen, Eugene, OR). For each labeling, a fresh stock of DiO was made by dissolving powder in N,N-dimethylformamide (DMF) at a concentration of 1 μg/μL, warmed to 50°C, and mixed with diluted leukoreduced murine RBCs (100 μL DiO stock into 1 mL of packed RBCs diluted in 10 × volume of modified phosphate-buffered saline [PBS; adjusted to 340 Osm with NaCl]). After labeling at 37°C for 30 minutes in the dark, the RBCs were washed 3 times, and samples were run on a flow cytometer to confirm labeling. DiO fluorescence was read on the channel normally used for measuring fluorescein, in this case “FL-1.”
Treatment of mice and transfusion of blood
A total of 6 μL DiO-labeled C57BL/6 RBCs, diluted in a total volume of 500 μL with PBS, were transfused into syngeneic recipients. The 6-μL amount was chosen for the DiO experiments after careful titration, as this is the amount that places APC consumption on the linear part of the curve for this assay, thus allowing the most sensitive quantitation. Syngeneic RBCs were used to avoid interference by any naturally occurring antibodies.
Mice were pretreated with intraperitoneal injections of poly (I:C) 4 hours prior to transfusion. Injections consisted of 100 μg poly (I:C) (Amersham, Piscataway, NJ) in 500 μL PBS. Control mice received injections of PBS. In some experiments, B10.BR mice were splenectomized (using standard murine surgical techniques) and transfused 3 weeks later with 100 μL leukoreduced, packed mHEL RBCs, diluted to a total volume of 500 μL with PBS.
Isolation of spleen, liver, and lymph nodes
Spleens were processed in Hanks balanced salt solution (HBSS; Cellgro; Mediatech, Herndon, VA) by grinding between frosted glass slides (Fisher Scientific, Hampton, NH), and RBCs were lysed with ammonium chloride (Sigma, St Louis, MO). In experiments involving adoptively transferred 3A9 × B6.PL-Thy1.1 CD4+ T cells, spleens were subjected to Spin Sep enrichment (StemCell Technologies, Vancouver, BC) of CD4+ T cells prior to analysis. Livers were incubated with collagenase (0.6 mg/mL; Worthington, Lakewood, NJ) in HBSS with 5% fetal bovine serum. Digested livers were then dispersed by grinding through a sieve, and APCs were then isolated by described methodologies.28,29 Lymph nodes were ground between frosted glass slides and washed in PBS, and RBCs were lysed. Organs were harvested at various time points (days 1-5) after transfusion or treatment.
Adoptive transfer of CD4+ T cells from T-cell-receptor transgenic mice
Adoptive transfer of CD4+ T cells from 3A9 × B6.PL-Thy1.1 into C57BL/6 × B10.BR mice was performed by tail-vein injection of 1.5 × 106 CD4+ T cells in 500 μL PBS. Prior to injection, CD4+ T cells were isolated using the Spin Sep system (Stem Cell Technologies), and were labeled with 5 μM CFSE (Invitrogen, Eugene, OR). At 48 hours after adoptive transfer of 3A9 × B6.PL-Thy1.1 CD4+ T cells, mice were treated intraperitoneally with 100 μg poly (I:C) or PBS, and then transfused intravenously with 100 μL leukoreduced, packed mHEL RBCs or C57BL/6 RBCs, diluted to a total volume of 500 μL with PBS.
Antibodies and flow cytometry
Antibodies to TER-119, CD80, CD86, and CD40 (including the appropriate isotype-matched controls) were purchased from BD Pharmingen (San Diego, CA). Antibodies to F4/80, CD11c, Ox40L, CD70, 4-1BBL, CD30L, and Thy1.1 (including the appropriate isotype-matched controls) were purchased from eBiosciences (San Diego, CA). All antibodies were directly conjugated. Cells were treated with Fc block (anti-mouse CD16/CD32; BD Pharmingen) prior to staining with the mentioned antibodies. All staining was performed in fluorescence-activated cell sorter (FACS) buffer (PBS + 0.2 mg/mL bovine serum albumin [Sigma] + 0.9 mg/mL EDTA [Sigma]).
ELISA for anti-HEL humoral response
Enzyme-linked immunosorbent assays (ELISAs) for anti-HEL IgG were performed on sera taken 2 weeks following transfusion (at a 1:50 dilution), as previously described.26
Results
Transfused RBCs are consumed predominantly by macrophages and not DCs in the spleens of noninflamed mice
To measure the consumption of transfused RBCs by APCs, we developed a novel in vivo methodology using RBCs labeled with the fluorescent-dye DiO. We have previously demonstrated that DiO-labeled RBCs have a normal circulatory lifespan when transfused into recipient mice.30 To adapt this technology to the measure of RBC consumption by APCs, spleens of mice previously transfused with DiO-labeled RBCs were harvested. The hypothesis being tested focuses on the initial immune response before any anti-RBC antibodies were formed. Thus, the studies were performed with syngeneic C57BL/6 RBC donors and recipients to ensure that naturally occurring antibodies did not interfere.
A total of 106 splenocytes were stained, with macrophages and DCs visualized by staining with anti-F4/80 and anti-CD11c (Figure 1A,B). Gates constituting positive staining for F4/80 and CD11c were established based upon isotype-matched controls (Figure 1C, D). Strongly positive F4/80 and CD11c populations were designated as representing macrophages and DCs, respectively. A total of 105 events were counted.
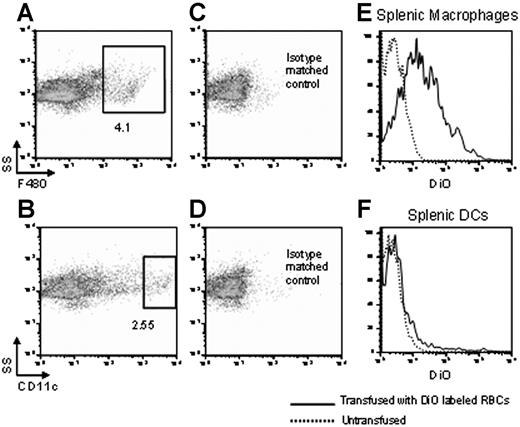
Transfused RBCs are consumed predominantly by macrophages and not DCs in the spleens of noninflamed mice. Syngeneic RBCs were labeled with DiO and transfused into recipient mice. Twenty-four hours later, splenocytes were harvested and either macrophages or DCs were visualized by staining with anti-F4/80 or anti-CD11c, respectively (A,B). Numbers represent percentage of cells in gate. Specificity was confirmed by staining with isotype matched controls (C,D). Numbers represent percentage of cells in gate. After gating on macrophages or DCs, DiO fluorescence was measured in each population (E,F) (–). Untransfused mice were used as a negative control to determine background fluorescence on the DiO channel (E,F) (------). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
Transfused RBCs are consumed predominantly by macrophages and not DCs in the spleens of noninflamed mice. Syngeneic RBCs were labeled with DiO and transfused into recipient mice. Twenty-four hours later, splenocytes were harvested and either macrophages or DCs were visualized by staining with anti-F4/80 or anti-CD11c, respectively (A,B). Numbers represent percentage of cells in gate. Specificity was confirmed by staining with isotype matched controls (C,D). Numbers represent percentage of cells in gate. After gating on macrophages or DCs, DiO fluorescence was measured in each population (E,F) (–). Untransfused mice were used as a negative control to determine background fluorescence on the DiO channel (E,F) (------). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
Consumption of transfused RBCs was determined by measuring DiO fluorescence in the gated populations. Untransfused mice were used as a negative control to establish baseline fluorescence on the channel that detects DiO. Splenic macrophages from mice transfused with DiO-labeled RBCs showed strong DiO fluorescence compared with macrophages from untransfused mice (Figure 1E). In contrast, significantly lower numbers of DCs consumed transfused RBCs (Figure 1F). Statistical analysis was done comparing the percentage of macrophages that were DiO+ to the percentage of DCs that were DiO+. A combined analysis of 3 of 3 experiments demonstrated that on average there was a mean 4.0-fold (95% confidence interval [95% CI], 2.6-5.4; SD, 1.6) greater number of macrophages that consumed RBCs compared with DCs.
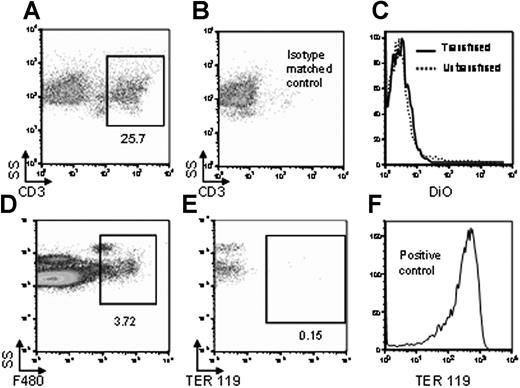
To test the possibility that DiO fluorescence of macrophages was an artifact of nonspecific dye transfer, nonphagocytic cells (T cells) were visualized by staining with anti-CD3 (Figure 2A). A CD3+ gate was established based upon staining with an isotype-matched control (Figure 2B). In contrast to macrophages, there was no increased DiO fluorescence in T cells from mice transfused with DiO-labeled RBCs compared with untransfused mice (Figure 2C). These data suggest that the observed fluorescence of the macrophages in mice transfused with DiO-labeled RBCs was not an artifact of nonspecific DiO transfer.
DiO fluorescence is not due to nonspecific transfer of DiO, nor to RBC adherence to the APC surface. Twenty-four hours after transfusion with DiO-labeled syngeneic RBCs, splenocytes were harvested, and nonphagocytic T cells were visualized by staining with anti-CD3 (A). Specificity was confirmed by staining with an isotype-matched control (B). After gating on CD3+ T cells, DiO fluorescence was measured both in mice transfused with DiO-labeled RBCs (–) or untransfused negative control mice (------) (C). In a separate control experiment, splenic macrophages were visualized by anti-F4/80 staining (D). After gating on the F4/80+ population, fluorescence of anti-TER-119 was analyzed (E). RBCs were stained with anti-TER-119 as positive control for antibody activity (F). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results. For panels A, B, D, E, numbers represent percentage of cells in gate.
DiO fluorescence is not due to nonspecific transfer of DiO, nor to RBC adherence to the APC surface. Twenty-four hours after transfusion with DiO-labeled syngeneic RBCs, splenocytes were harvested, and nonphagocytic T cells were visualized by staining with anti-CD3 (A). Specificity was confirmed by staining with an isotype-matched control (B). After gating on CD3+ T cells, DiO fluorescence was measured both in mice transfused with DiO-labeled RBCs (–) or untransfused negative control mice (------) (C). In a separate control experiment, splenic macrophages were visualized by anti-F4/80 staining (D). After gating on the F4/80+ population, fluorescence of anti-TER-119 was analyzed (E). RBCs were stained with anti-TER-119 as positive control for antibody activity (F). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results. For panels A, B, D, E, numbers represent percentage of cells in gate.
To address the possibility that DiO fluorescence of macrophages was due to intact RBCs adhering to the macrophage surface, splenic preparations were stained with anti-F4/80 and anti-TER-119, the latter being specific for murine RBCs. After gating on F4/80+ macrophages (Figure 2D), no positive staining with anti-TER-119 was observed (Figure 2E), demonstrating that the observed DiO fluorescence was not due to RBC adherence to the external macrophage surface. This result was not due to a lack of anti-TER-119 staining activity, as intact RBCs stain strongly positive for TER-119 (Figure 2F).
Labeling of RBCs with DiO requires ex vivo manipulation of the RBCs. Because DiO-labeled RBCs have a normal lifespan when transfused into recipient mice,30 it is unlikely that the labeling procedure significantly alters patterns of RBC clearance. However, it is possible that a small percentage of RBCs are damaged during the DiO labeling, and that these damaged cells may be rapidly cleared upon transfusion in a different pattern than normal RBC clearance. To control for this possibility, DiO-labeled RBCs were transfused into mice and allowed to circulate for 24 hours to allow for the removal of any damaged cells. The mice were then exsanguinated, and the blood was promptly transfused into new mice with minimal manipulation. Spleens were then harvested from the second recipients and subjected to analysis for DiO consumption by macrophages and DCs as described. The use of secondary transfusions gave similar results as in the primary animals (data not shown), suggesting that DiO fluorescence in macrophages was not an artifact of phagocytosis of particles from RBCs damaged during the DiO labeling.
Together, these data demonstrate that in the spleens of otherwise unmanipulated mice, transfused RBCs are consumed predominantly by macrophages, with only trace consumption by DCs.
Consumption of transfused RBCs by APCs in liver and lymph nodes
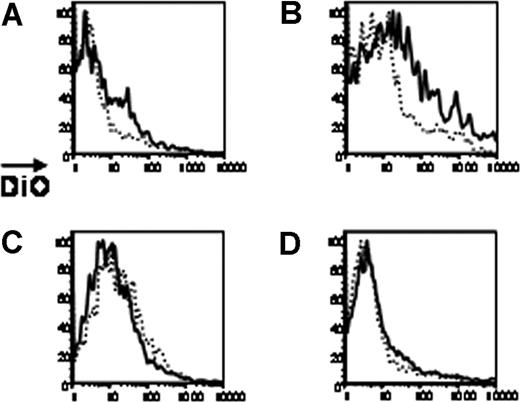
Using this approach with syngeneic RBCs, the consumption of DiO-labeled RBCs by APCs in liver and lymph nodes was assessed. In contrast to the spleen, only low levels of DiO fluorescence were observed in liver macrophages (Figure 3A). Moreover, hepatic DCs consumed more RBCs than hepatic macrophages (Figure 3B). Statistical analysis was done comparing the percentage of hepatic DCs that were DiO+ to the percentage of macrophages that were DiO+. A combined analysis of 3 of 3 experiments demonstrated that hepatic DCs consumed a mean 3.3-fold more RBCs than did hepatic macrophages. However, there was variability from mouse to mouse, with a 95% CI of 0.5 to 6.1 and an SD of 3.2. Nevertheless, the overall levels of consumption of RBCs in the liver are an order of magnitude lower than that observed in the spleen, and the pattern of consumption by macrophages versus DCs is different.
Consumption of RBCs by liver and lymph node APCs. Syngeneic RBCs were labeled with DiO and transfused into recipient mice. Twenty-four hours after transfusion, livers and lymph nodes were processed. Macrophages or DCs were visualized by staining with anti-F4/80 or anti-CD11c (not shown) After gating on liver macrophages (A), liver DCs (B), lymph node macrophages (C), or lymph node DCs (D), DiO fluorescence was measured in each populations (–). Untransfused mice were used as a negative control for background DiO fluorescence (------). Staining from representative mice is shown, and y-axes of histograms represent percentages of maximum peak value. This experiment was performed 3 times with similar results.
Consumption of RBCs by liver and lymph node APCs. Syngeneic RBCs were labeled with DiO and transfused into recipient mice. Twenty-four hours after transfusion, livers and lymph nodes were processed. Macrophages or DCs were visualized by staining with anti-F4/80 or anti-CD11c (not shown) After gating on liver macrophages (A), liver DCs (B), lymph node macrophages (C), or lymph node DCs (D), DiO fluorescence was measured in each populations (–). Untransfused mice were used as a negative control for background DiO fluorescence (------). Staining from representative mice is shown, and y-axes of histograms represent percentages of maximum peak value. This experiment was performed 3 times with similar results.
RBCs are not typically thought to enter lymphatic fluid. However, as the lymph nodes are vascularized tissues, it was unclear if transfused RBCs would be consumed by lymphatic APCs. Analysis of the lymph nodes demonstrated a lack of DiO fluorescence in either macrophages or DCs from transfused mice, suggesting essentially no consumption of transfused RBCs by APCs in peripheral lymph nodes (Figure 3C, D).
Changes in patterns of RBC consumption by APCs in response to inflammation with poly (I:C)
We have previously reported that inflammation of recipient mice with poly (I:C) significantly increases both the frequency and the magnitude of alloimmunization to a foreign antigen on transfused RBCs.26 We hypothesized that poly (I:C) was acting by increasing the immunogenicity of APCs. To test this hypothesis, consumption of transfused RBCs was assessed in mice inflamed with poly (I:C). As stated, syngeneic RBCs were transfused in order to avoid any potentially interfering antibodies. In the presence of poly (I:C), there was a significant increase in consumption of transfused RBCs by splenic DCs. A combined analysis of 3 of 3 experiments demonstrated that splenic DCs from mice treated with poly (I:C) had a mean 2.4-fold increase (95% CI, 1.7-3.1; SD, 0.8) in consumption of RBCs compared with splenic DCs from noninflamed mice (Figure 4A shows a representative experiment). Increased consumption in response to poly (I:C) was also seen in the liver DCs; hepatic DCs from mice treated with poly (I:C) had a mean 1.9-fold increase (95% CI, 1.7-2.1; SD, 0.3) in consumption of RBCs compared with hepatic DCs from noninflamed mice (Figure 4B shows a representative experiment). In contrast, a slight decrease in consumption (mean 0.7-fold decrease; 95% CI, 0.6-0.8; SD, 0.1) was seen by splenic macrophages in the presence of poly (I:C) (Figure 4C shows a representative experiment), with a subtle increase in consumption seen by liver macrophages (mean 3.0-fold increase; 95% CI, 1.2-4.8; SD, 2.0; Figure 4D shows a representative experiment).
Inflammation with poly (I:C) enhances consumption of transfused RBCs by DCs. Syngeneic RBCs were labeled with DiO and transfused into recipient mice that were preinjected intraperitoneally with either poly (I:C) or control PBS. Twenty-four hours after transfusion, spleens and livers were harvested. Macrophages or DCs were visualized by staining with anti-F4/80 or anti-CD11c (not shown). DiO fluorescence was measured after gating on DCs from the spleen (A) or liver (B), or on macrophages from the spleen (C) or liver (D). Cells from mice treated with poly (I:C) are solid lines, whereas control animals are dotted lines. Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
Inflammation with poly (I:C) enhances consumption of transfused RBCs by DCs. Syngeneic RBCs were labeled with DiO and transfused into recipient mice that were preinjected intraperitoneally with either poly (I:C) or control PBS. Twenty-four hours after transfusion, spleens and livers were harvested. Macrophages or DCs were visualized by staining with anti-F4/80 or anti-CD11c (not shown). DiO fluorescence was measured after gating on DCs from the spleen (A) or liver (B), or on macrophages from the spleen (C) or liver (D). Cells from mice treated with poly (I:C) are solid lines, whereas control animals are dotted lines. Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
Effects of poly (I:C) on costimulatory molecule expression on APCs
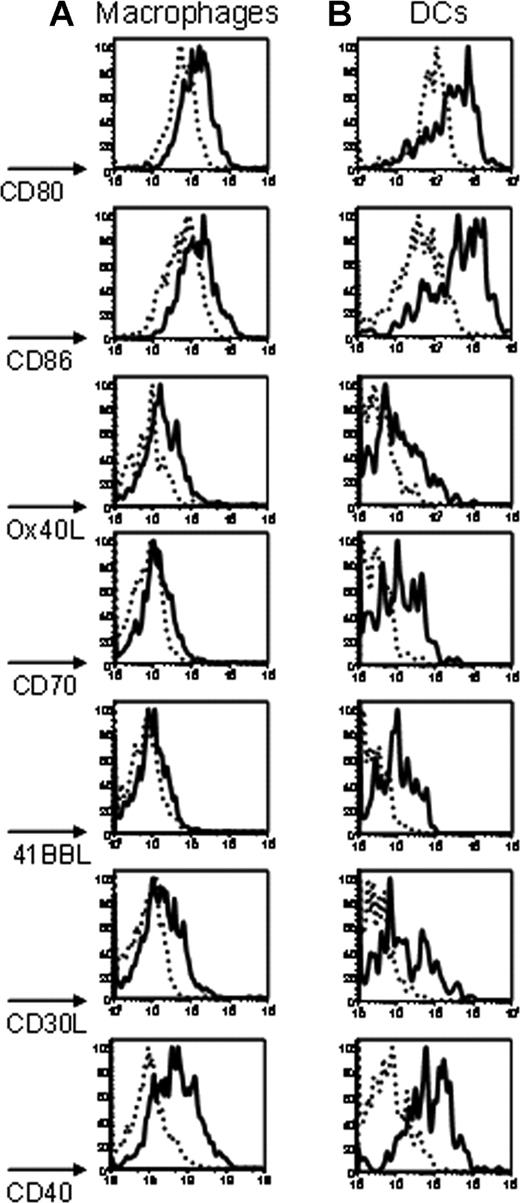
To determine the effect of poly (I:C) on the expression of costimulatory molecules, macrophages and DCs were stained with a panel of antibodies against known costimulatory molecules (CD80,31 CD86,32 Ox40L,33 CD70,33 4-1BBL,34 CD30L,35 and CD4036 ). On macrophages, treatment with poly (I:C) induced an increased expression of CD80, CD86, Ox40L, CD30L, and CD40 (Figure 5A). Only a slight increase was observed for CD70 and 41BBL (Figure 5A). In contrast, a more substantial increase in expression was observed for all of the examined costimulatory molecules on DCs (Figure 5B). Kinetics analysis demonstrated that induction of costimulatory molecule expression began by 6 hours after injection with poly (I:C), peaked at approximately 24 hours, and returned to baseline by 3 days (data not shown). Similar findings were observed in mice receiving poly (I:C) treatment followed by transfusion with mismatched (mHEL) RBCs (data not shown).
Poly (I:C) induces co-stimulatory molecule expression on macrophages and DCs. Twenty-four hours after intraperitoneal injection with either poly (I:C) or control PBS, splenocytes were harvested, and macrophages and DCs were visualized with anti-F4/80 or anti-CD11c, respectively (not shown). After gating on macrophages (A) or DCs (B), staining with antibodies specific for the indicated costimulatory molecules was performed. Cells from mice treated with poly (I:C) (–) are compared with control mice injected with PBS (------). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
Poly (I:C) induces co-stimulatory molecule expression on macrophages and DCs. Twenty-four hours after intraperitoneal injection with either poly (I:C) or control PBS, splenocytes were harvested, and macrophages and DCs were visualized with anti-F4/80 or anti-CD11c, respectively (not shown). After gating on macrophages (A) or DCs (B), staining with antibodies specific for the indicated costimulatory molecules was performed. Cells from mice treated with poly (I:C) (–) are compared with control mice injected with PBS (------). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
APCs presenting RBC alloantigens are more potent in the presence of poly (I:C)
The data presented thus far demonstrate that poly (I:C) induces increased consumption of transfused syngeneic RBCs and increased expression of costimulatory molecules by recipient APCs. Based upon this, we hypothesized that the recipient APCs would be more potent at initiating an immune response against an RBC alloantigen. To test this hypothesis, we used mHEL mice, which are transgenic for mHEL and express mHEL on the surface of RBCs.30 Thus, mHEL represents a model RBC alloantigen present in donor but not recipient mice. We also used 3A9 mice, which are transgenic for a T-cell receptor that recognizes the immunodominant epitope of HEL presented by I-Ak.27 The 3A9 mice were crossed with C57BL/6 mice that are congenic for Thy1.1 (B6.PL-Thy1.1). CD4+ T cells were isolated from 3A9 × B6.PL-Thy1.1 mice and were adoptively transferred into C57BL/6 × B10.BR mice. In this way, both animals were on an identical H-2 b × H-2k background, and the 3A9 × B6.PL-Thy1.1 cells could be visualized by the Thy1.1 molecule. Prior to adoptive transfer, the 3A9 × B6.PL-Thy1.1 cells were labeled with the fluorescent dye CFSE. As cells divide, CFSE fluorescence is equally distributed to daughter cells, allowing for an analysis of cellular division as a function of decreased CFSE fluorescence.37 The C57BL/6 × B10.BR mice receiving the adoptively transferred 3A9 × B6.PL-Thy1.1 cells were then treated with poly (I:C) or PBS and were transfused with leukoreduced mHEL or control C57BL/6 RBCs. A volume of 100 μL of packed RBCs was transfused in a total volume of 500 μL, an amount equivalent to approximately 1 unit of RBCs in a human.
At 3 days after transfusion, splenocytes were harvested and CD4+ cells were enriched by negative selection. The adoptively transferred 3A9 × B6.PL-Thy1.1 cells were visualized by staining with anti-Thy1.1 coupled to allophycocyanin (with 2 × 106 CD4+ cells being counted on the flow cytometer). An example of the visualized cells are shown (Figure 6A). Only minimal cells were seen in this same gate in mice not adoptively transferred with 3A9 × B6.PL-Thy1.1 cells (data not shown). After gating on the Thy1.1+ cells, division was assessed by quantifying the decrease in CFSE fluorescence.37 No division was observed in control mice that received C57BL/6 RBCs that lacked the mHEL RBC alloantigen. Division was observed in mice that received mHEL RBCs, and this division was significantly enhanced in mice that were inflamed with poly (I:C) prior to transfusion with mHEL RBCs. A combined analysis of 3 of 3 experiments (with a representative shown in Figure 6B) demonstrated a CFSE median fluorescence intensity of 3A9 × B6.PL-Thy1.1 CD4+ cells that was 4.7-fold lower in the presence of poly I:C compared with that in the absence of poly (I:C) (95% CI, 2.0-7.4). To confirm that adoptive transfer of 3A9 × B6.PL-Thy1.1 cells did not alter the biology being studied, sera were collected from parallel animals and ELISAs measuring anti-HEL IgG were performed at later time points. Under these conditions, poly (I:C) still enhanced alloimmunization to mHEL RBCs (data not shown). Together, these data demonstrate that treatment with poly (I:C) results in enhanced proliferation of CD4+ T cells specific for an alloantigen on transfused RBCs.
Increased division of antigen-specific CD4+ T cells in the presence of poly (I:C), following transfusion with mHEL RBCs. After adoptive transfer of CFSE-labeled antigen-specific 3A9 × B6.PL-Thy1.1 CD4+ T cells, recipients were transfused with mHEL or control C57BL/6 RBCs, in the presence or absence of inflammation with poly (I:C). Three days later, splenocytes were analyzed for division of 3A9 × B6.PL-Thy1.1 cells. (A) Gating on Thy1.1+ adoptively transferred 3A9 × B6.PL-Thy1.1 cells. Numbers represent percentage of cells in gate. (B) CFSE division of the antigen-specific CD4+ T-cell population (gray shaded histogram is C57BL/6 control; dotted-line histogram is transfused with mHEL; and solid-line histogram is transfused with mHEL in the presence of poly (I:C)). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
Increased division of antigen-specific CD4+ T cells in the presence of poly (I:C), following transfusion with mHEL RBCs. After adoptive transfer of CFSE-labeled antigen-specific 3A9 × B6.PL-Thy1.1 CD4+ T cells, recipients were transfused with mHEL or control C57BL/6 RBCs, in the presence or absence of inflammation with poly (I:C). Three days later, splenocytes were analyzed for division of 3A9 × B6.PL-Thy1.1 cells. (A) Gating on Thy1.1+ adoptively transferred 3A9 × B6.PL-Thy1.1 cells. Numbers represent percentage of cells in gate. (B) CFSE division of the antigen-specific CD4+ T-cell population (gray shaded histogram is C57BL/6 control; dotted-line histogram is transfused with mHEL; and solid-line histogram is transfused with mHEL in the presence of poly (I:C)). Staining from representative mice is shown, and y-axes of histograms represent percentage of maximum peak value. This experiment was performed 3 times with similar results.
The spleen is required for the enhancing effects of poly (I:C) on RBC alloimmunization
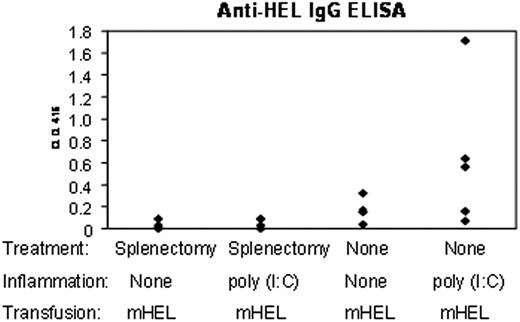
These data demonstrated that inflammation with poly (I:C) increased consumption of transfused syngeneic RBCs by splenic DCs, with a concomitant increase in costimulatory molecule expression. Enhancement of CD4+ T-cell proliferation, specific for the mHEL antigen, was also seen after transfusion of mismatched RBCs in the presence of poly (I:C). To test if the splenic APCs are required for enhanced RBC alloimmunization, a subset of mice were splenectomized prior to transfusion. Splenectomized and nonsplenectomized mice were transfused with leukoreduced RBCs from mHEL donor mice in the absence or presence of inflammation with poly (I:C). To assess alloimmunization, sera were collected 2 weeks after immunization, and anti-HEL IgG was determined using an anti-HEL ELISA.
As we have previously described, poly (I:C) significantly enhanced RBC alloimmunization to mHEL (Figure 7). However, this effect was abrogated by splenectomy. Enhancement of alloimmunization by poly (I:C) for each experiment was defined as anti-HEL IgG levels in poly (I:C)-treated mice that exceeded 2 standard deviations of the mean anti-HEL IgG levels found in noninflamed mice. Combined data from 3 of 3 experiments (with a representative experiment shown in Figure 7), demonstrated that poly (I:C) caused enhancement of anti-HEL IgG production in 9 of 14 nonsplenectomized mice (with a mean fold enhancement among enhancers of 4.7 [95% CI, 3.1-6.3; SD, 2.4]). In contrast, inflammation enhanced alloimmunization to mHEL RBCs in only 2 of 14 splenectomized mice. However, it is also worth noting that splenectomy decreased alloimmunization even in the absence of inflammation. Together, these data demonstrate that splenic tissues play an important role in both RBC alloimmunization to the mHEL antigen and in the enhancement of alloimmunization by poly (I:C).
Poly (I:C) does not enhance alloimmunization to mHEL RBCs in the absence of a spleen. Splenectomized and nonsplenectomized mice were treated intraperitoneally with poly (I:C) or control PBS injections. Following treatment, mice were transfused with leukoreduced mHEL RBCs. Two weeks after transfusion, anti-HEL responses were measured using an anti-HEL IgG ELISA. This experiment was performed 3 times with similar results.
Poly (I:C) does not enhance alloimmunization to mHEL RBCs in the absence of a spleen. Splenectomized and nonsplenectomized mice were treated intraperitoneally with poly (I:C) or control PBS injections. Following treatment, mice were transfused with leukoreduced mHEL RBCs. Two weeks after transfusion, anti-HEL responses were measured using an anti-HEL IgG ELISA. This experiment was performed 3 times with similar results.
Discussion
Herein, we demonstrate that in the noninflamed state, transfused syngeneic RBCs are consumed predominantly by splenic macrophages, with only trace consumption by DCs. Lower levels of consumption take place by macrophages and DCs in the liver, and essentially no consumption occurs in peripheral lymph nodes. These findings underscore the somewhat unique nature of APC consumption of transfused RBC antigens as opposed to antigens from purified proteins or microbial infection, which typically drain into peripheral nodes.
The demonstrated pattern of APC consumption also suggests that under noninflamed conditions, RBCs are selectively shunted into compartments that may be only weakly immunogenic or nonimmunogenic. In many contexts, macrophages are substantially weaker APCs than are DCs.38 Although some transfused RBCs are consumed by DCs in the liver, there is evidence that the hepatic environment may be immunosuppressive, and hepatic APCs are poor initiators of immunity that may even promote tolerance.39,40 Together, our data indicate that, in the absence of inflammation, transfused syngeneic RBCs are selectively consumed by subsets of APCs that may be poor initiators of immunity and/or promote tolerance. Overall, these findings provide novel insights into the unique nature of RBCs as immunogens, and suggest one reason why RBC alloimmunization rates are usually low.
In contrast to the quiescent state, inflammation with poly (I:C) induces consumption of transfused RBCs by splenic DCs, with a slight decrease in consumption by splenic macrophages. Consumption of transfused RBCs by DCs is also increased in the liver. Moreover, poly (I:C) induces increased expression of a large number of costimulatory molecules, more so on DCs than macrophages, which are well understood to result in potent stimulation of a primary immune response.41 These processes would be expected to occur even in the absence of transfusion, and thus may also contribute to potential induction of autoimmunization by inflammation. Last, following transfusion with mismatched mHEL RBCs, in vivo proliferation of CD4+ T cells specific for the RBC alloantigen is enhanced by poly (I:C), suggesting functional significance to the observed APC changes that results in increased activation of naive CD4+ T cells. Thus, we conclude that inflammation with poly (I:C) enhances RBC alloimmunization by simultaneously shunting RBC antigens into a more immunogenic APC type and significantly increasing expression of costimulatory molecules on those APCs.
Poly (I:C) fails to enhance RBC alloimmunization in splenectomized mice, suggesting that the targets of poly (I:C) enhancement are in the spleen. Although this is consistent with the interpretation given here, other cellular components of the immune system, upon which poly (I:C) may act, are also contained in the spleen. Moreover, even in the noninflamed state, splenectomized mice have less RBC alloimmunization than nonsplenectomized mice. Thus, it is also possible that the spleen is simply required for full alloimmunization to the mHEL RBC antigen, and that poly (I:C) enhances at a different site that requires the spleen in order to function.
Although the current data support a role of poly (I:C) in enhancing RBC alloimmunization at the level of the APC, these findings do not exclude additional effects of poly (I:C) at different points in immunization. Indeed, poly (I:C) is also known to have considerable effects on natural killer (NK) cells,42 direct effects on CD4+ T cells,43 and also to result in induction of high levels of immunomodulatory cytokines,44 such as interferons. In addition, poly (I:C) activates other double-stranded RNA-responsive molecules, such as the double-stranded RNA-dependent kinase (PKR) and 2′5′-oligoadenylate synthetase45 . Thus, it is possible that the observed enhanced proliferation of CD4+ T cells is also a function of direct effects on the CD4+ T cell or indirect effects from cytokine stimulation. Ongoing studies are addressing these possibilities.
It must be remembered that poly (I:C) is only one type of inflammation, and mHEL is only one model of RBC alloantigen. Thus, it is unclear to what extent these findings may be generalized to RBC alloimmunization. Of note, it has recently been reported that inducing inflammation with CpG oligodeoxynucleotide likewise enhanced RBC alloimmunization (in a different murine model that uses human glycophorin A on mouse RBCs as an alloantigen).46 Moreover, like our previous findings with mHEL and poly (I:C), little or no alloimmunization occurred in the absence of CpG despite repeated transfusion. Thus, in at least 2 different mouse models, inflammation appears to play a role in RBC alloimmunization.
The current studies focus on mechanisms underlying the role that inflammation may play in alloimmunization to transfused RBCs. The source of inflammation during transfusion may come from the pathology of the recipient; alternatively, even in the case that there is no obvious recipient inflammation, mediators of inflammation may be found in the donor unit. However, inflammation is likely just one component of a process (RBC alloimmunization) that is affected by multiple variables,47 including (1) dose of antigen, (2) differences in blood group antigens between donor and recipient, and (3) recipient genetics (ie, HLA type).12,13 Thus, the current investigation provides insight into what is likely one factor in the overall regulation of RBC alloimmunization.
In summary, the current findings provide novel insight into the nature of RBCs as an immunologic stimulus. These data support a model in which transfused RBCs are shunted to weakly immunogenic or nonimmunogenic compartments in the absence of inflammation. In contrast, inflammation results in a shifting of RBC consumption patterns toward more immunogenic DCs with a concomitant increase in costimulatory molecule expression, leading to a significant increase in division of antigen-specific CD4+ T cells. These findings add to the understanding of the mechanisms behind RBC alloimmunization, and may lead to targeted immunomodulatory strategies to prevent RBC alloimmunization.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors would like to thank Kirk Easley of the Department of Biostatistics, Rollins School of Public Health, Emory University, for his statistical assistance.
This work was supported in part by grants from the National Institutes of Health (HL069769) and the National Blood Foundation (to J.E.H.).
National Institutes of Health
Authorship
Contribution: J.E.H. and J.C.Z. designed the research, analyzed and interpreted the data, and drafted the manuscript. J.E.H. and T.E.C. performed the research. J.D.R. and C.D.H. analyzed and interpreted the data and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James C. Zimring, Center for Transfusion and Cellular Therapies, Department of Pathology and Laboratory Medicine, Emory University School of Medicine, Woodruff Memorial Building Suite 7301, 101 Woodruff Cir, Atlanta, GA 30322; e-mail:jzimrin@emory.edu.