IgE/antigen-dependent mast cell activation plays a central role in immediate hypersensitivity and other allergic reactions. The Src family tyrosine kinase (SFK) Lyn is activated by the cross-linking of high-affinity IgE receptors (FcϵRI). Activated Lyn phosphorylates the FcϵRI subunits, β and γ, leading to subsequent activation of various signaling pathways. Lyn also plays a negative regulatory function by activating negative regulatory molecules. Another SFK, Fyn, also contributes to mast cell degranulation by inducing Gab2-dependent microtubule formation. Here we show that a third SFK, Hck, plays a critical role in mast cell activation. Degranulation and cytokine production are reduced in FcϵRI-stimulated hck−/− mast cells. The reduced degranulation can be accounted for by defects in Gab2 phosphorylation and microtubule formation. Importantly, Lyn activity is elevated in hck−/− cells, leading to increased phosphorylation of several negative regulators. However, positive regulatory events, such as activation of Syk, Btk, JNK, p38, Akt, and NF-κB, are substantially reduced in hck−/− mast cells. Analysis of lyn−/−hck−/−, lyn−/−FcϵRIβ−/−, and hck−/−FcϵRIβ−/− cells shows that Hck exerts these functions via both Lyn-dependent and Lyn-independent mechanisms. Thus, this study has revealed a hierarchical regulation among SFK members to fine-tune mast cell activation.
Introduction
Mast cells are key effector cells for IgE-dependent immediate hypersensitivity and other allergic reactions. These reactions are triggered by cross-linking of the high-affinity IgE receptor, FcϵRI, with IgE and multivalent antigen. FcϵRI consists of an IgE-binding α subunit, a signal-amplifying tetramembrane-spanning β subunit, and 2 signal-generating γ subunits.1 According to the widely accepted model,2 the following events occur on receptor aggregation: FcϵRIβ-associated Lyn, a Src family protein-tyrosine kinase (PTK), phosphorylates tyrosine residues of the immunoreceptor tyrosine-based activation motifs (ITAMs) in β and γ subunits.3,4 The phosphorylated ITAMs in the β and γ subunits recruit Lyn and Syk molecules, respectively.4 These ITAM-bound PTKs phosphorylate a multitude of signaling proteins, leading to the activation of several signaling pathways including phosphatidylinositol 3-kinase, phospholipase C/Ca2+, and mitogen-activated protein kinases.2,5,–7 In addition to Lyn, recent studies suggested the presence of another pathway required for degranulation: Fyn, another FcϵRI-associated Src family tyrosine kinase (SFK), mediates phosphorylation of the adaptor protein Gab2, leading to phosphatidylinositol 3-kinase activation,8 as well as Ca2+-independent microtubule formation.9 Concerted action of these pathways leads to degranulation (release of preformed vasoactive amines and other proinflammatory mediators), synthesis and release of leukotrienes and their derivatives, and production and secretion of cytokines.
In addition to its signal-initiating activity through phosphorylation of tyrosine residues in the γ-ITAM, Lyn also plays a negative regulatory role in aspects of mast cell activation10,,,–14 : IgE/mast cell-dependent in vivo anaphylactic reactions are enhanced in young lyn−/− mice.12 FcϵRI stimulation induces greater production of cytokines in lyn−/− than in wild-type (WT) mast cells,10 whereas the same stimulation induces reduced degranulation in mast cells expressing a constitutively active Lyn.14 The β subunit also plays both positive and negative regulatory roles in mast cell activation.15,,,,–20 In mouse mast cells, the β subunit is required for stable surface expression of FcϵRI. The β-ITAM is unique in 2 ways: the spacing between the 2 canonical tyrosines (Tyr-219 and Tyr-229) harbors a third tyrosine (Tyr-225), and it is one amino acid shorter than canonical ITAMs, making it unfit to bind the tandem SH2 domains of Syk, a PTK essential for most, if not all, activation outcomes.21,22 Phosphorylation of Tyr-219 contributes to the enhancement of mast-cell activation, whereas that of Tyr-225 opposes it.19,20 Lyn can phosphorylate both Tyr-219 and Tyr-225 residues, and therefore its roles in positive and negative regulation of mast cell activation are exerted at least in part through phosphorylation of β-ITAM residues. Our recent study showed that Lyn plays a positive regulatory role in survival, degranulation, and cytokine production when mast cells were stimulated with “low-intensity” stimuli such as IgE+ low-concentration antigen (Ag) and IgE+ anti-IgE, whereas these activation events were negatively regulated by Lyn on “high-intensity” stimulation with IgE+ high-concentration Ag.23 Lyn appears to use its associated protein, FcϵRIβ, as a pivotal molecule to negatively regulate downstream events on the latter stimulation, because “low-intensity” stimuli leads to a dissociation of Lyn with FcϵRIβ, but “high-intensity” stimuli leads to an increased association of Lyn with FcϵRIβ.23 “High-intensity” or supraoptimal antigen stimulation results in the downward phase of bell-shaped antigen dose-response curves. Suppression of mast-cell activation in this phase depends on Lyn,11 Src homology-2-containing inositol 5′-phosphatase (SHIP),24 protein kinase C-δ,25 and the actin cytoskeleton.26,–28
Here we show that Hck plays a positive regulatory role in mast- cell activation induced under “high-intensity” FcϵRI stimulation, in part by suppressing the negative regulatory Lyn kinase activity. In contrast, Lyn-mediated inhibitory signaling does not work under “low-intensity” stimulation.
Materials and methods
Sources of antibodies and some procedures are given in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Mice, cell culture, and FcϵRI stimulation
Bone marrow cells from WT and mutant mice were cultured in IL-3 for 4 to 6 weeks to generate bone marrow mast cells (BMMC) with more than 95% purity (c-Kit+ FcϵRI+). Lyn−/−,29 hck−/−,30 and FcϵRIβ−/−31 mice were used. These mice were backcrossed to C57BL/6 mice for at least 8 generations. lyn−/−hck−/−, lyn−/−FcϵRIβ−/−, and hck−/−FcϵRIβ−/− double-knockout mice were generated by crossing single-knockout mice. For FcϵRI stimulation, BMMCs were first sensitized by a 24-hour incubation with 0.5 μg/mL of H1 DNP-ϵ-206 IgE. BMMCs were washed and stimulated with the indicated concentrations of antigen, DNP23-HSA.
Measurements of histamine and cytokines
Amounts of histamine secreted from BMMCs were measured as described.32 Supernatants of BMMCs were measured by enzyme-linked immunosorbent assay for IL-6 and tumor necrosis factor-α (BD Biosciences Pharmingen, San Diego, CA).
Ca2+ measurement
IgE-sensitized BMMCs were loaded with Indo 1-AM (Calbiochem, San Diego, CA) and stimulated with various concentrations of antigen. Fluorescence ratio (525:405 nm) was continuously measured using flow cytometer BD-LSR, as described previously.33
Immunoblotting and in vitro kinase assays
Immunoblotting with or without immunoprecipitation and kinase assays for Lyn, Fyn, Syk and JNK molecules were performed as described previously.33
Retroviral transduction
Retroviral transduction of lyn−/−hck−/−, lyn−/−FcϵRIβ−/−, and hck−/−FcϵRIβ−/− mast cells was performed as described previously.34 Briefly, pMX-puro plasmids harboring WT or kinase-dead mouse hck cDNA, or WT (YYY) or mutant FcϵRI β cDNAs20 were transfected into packaging cells to generate recombinant retroviruses. BMMCs in culture media containing IL-3 and stem-cell factor (SCF) were infected with the viruses. Mass populations of puromycin-resistant cells were used for FcϵRI stimulation.
Microscopy
Slides were viewed with a Zeiss Axiovert Zoom inverted microscope (Carl Zeiss MicroImaging, Gottingen, Germany) using a Zeiss W-Pi Lens at 10×/23 and Zeiss Plan-Neofluar lens at 40×/1.3 and ProLong Gold antifade reagent with DAPI (Invitrogen, Eugene, OR). Images were acquired using a Photometrics Cool Snap HQ2 camera (Intelligent Imaging Innovations, Denver, CO), and were processed with Slidebook version 4.1 (Intelligent Imaging Innovations), and Adobe Illustrator version CS2 software (Adobe Systems, San Jose, CA).
Results
Hck protein is 30- to 50-fold less abundant than Lyn protein in mast cells
We determined the amount of 3 SFKs, Lyn, Fyn, and Hck, expressed in BMMCs by immunoblot analysis, using as a reference predetermined amounts of recombinant glutathione-S-transferase (GST)-tagged fusion proteins that contain the antigenic sequences of N-terminal unique regions of SFKs. As expected, Lyn was the most abundant SFK, with its p53lyn isoform present at approximately 500 ng/mg total cellular protein, whereas p56lyn was present at approximately 200 ng/mg (Figure 1C). The amount of p59fyn was estimated as 30 ng/mg. The amounts of p59hck and p56hck isoforms were estimated as low as 10 and 15 ng/mg, respectively (Figure 1B,C). Expression of Hck proteins was comparable in WT and lyn−/− BMMCs.
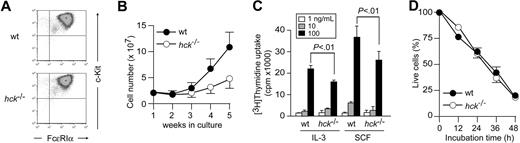
Hck deficiency results in reduced mast cell proliferation. (A) Flow cytometric analysis of FcϵRI and c-Kit expression on the surface of WT and hck−/− BMMCs. (B) Growth curves of bone marrow cells cultured in IL-3–containing medium. (C) Proliferation of WT and hck−/− BMMCs in response to the indicated concentrations of IL-3 or SCF were measured by thymidine uptake. Error bars represent standard deviation (SD) unless otherwise mentioned. (D) Growth factor-deprivation–induced apoptosis in WT and hck−/− BMMCs. Percentages of annexin V−/7AAD− live cells are plotted as a function of incubation time. Representative results from at least 3 independent experiments are shown.
Hck deficiency results in reduced mast cell proliferation. (A) Flow cytometric analysis of FcϵRI and c-Kit expression on the surface of WT and hck−/− BMMCs. (B) Growth curves of bone marrow cells cultured in IL-3–containing medium. (C) Proliferation of WT and hck−/− BMMCs in response to the indicated concentrations of IL-3 or SCF were measured by thymidine uptake. Error bars represent standard deviation (SD) unless otherwise mentioned. (D) Growth factor-deprivation–induced apoptosis in WT and hck−/− BMMCs. Percentages of annexin V−/7AAD− live cells are plotted as a function of incubation time. Representative results from at least 3 independent experiments are shown.
Hck positively regulates the proliferation of mast cells
To investigate the role of Hck in mast cells, bone marrow cells from WT and hck−/− mice were cultured in the presence of IL-3. Four weeks later, more than 95% pure populations of mast cells were generated as determined by flow cytometry for cell-surface expression of c-Kit and FcϵRI (Figure 1A), showing no significant differences between WT and hck−/− mice. Microscopic analysis of toluidine blue–stained cells revealed an indistinguishable metachromatic cell morphology (data not shown). Therefore, Hck deficiency does not seem to affect the mast-cell differentiation program. This notion was further supported by our observation showing that mast-cell numbers in the ear, back skin, stomach, and small intestine were not different between WT and hck−/− mice (data not shown).
However, culturing bone marrow cells from hck−/− mice in IL-3–containing medium yielded only approximately one-third of the number of mast cells derived from WT mice (Figure 1B). Thymidine uptake experiments indicated that proliferation of hck−/− mast cells in response to either IL-3 or SCF was significantly reduced compared with WT cells (Figure 1C). In contrast, growth factor deprivation induced comparable levels of apoptosis in both WT and hck−/− mast cells (Figure 1D). Taken together, these results demonstrate that Hck positively regulates proliferation, but not development or survival, of mast cells.
Hck deficiency leads to impaired FcϵRI-mediated degranulation and cytokine production
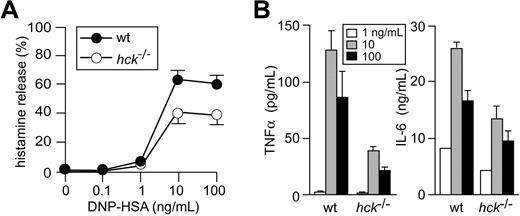
Degranulation and secretion of inflammatory mediators including various cytokines are cardinal features of FcϵRI-induced mast-cell activation. Histamine release (as a surrogate marker for degranulation) was significantly reduced in hck−/− mast cells stimulated with 10 or 100 ng/mL DNP23-HSA, compared with WT cells (Figure 2A). Hck deficiency drastically affected FcϵRI-induced tumor necrosis factor-α production: a 3- to 4-fold reduction was observed when anti-DNP IgE-sensitized mast cells were stimulated with 10 or 100 ng/mL DNP23-HSA (Figure 2B). IL-6 production was also reduced by Hck deficiency, albeit to a lesser extent. Therefore, these results show that Hck impacts on FcϵRI-mediated activation by playing a positive regulatory role, particularly when cells are stimulated with high concentrations of antigen.
Hck deficiency results in reduced histamine release and cytokine production when mast cells are stimulated with high concentrations of antigen. IgE-sensitized WT and hck−/− BMMCs were stimulated with the indicated concentrations of antigen for 45 minutes (A) or 20 hours (B). Histamine, tumor necrosis factor-α, and IL-6 secreted into culture media were measured. Representative results from 3 experiments are shown. Error bars represent SD.
Hck deficiency results in reduced histamine release and cytokine production when mast cells are stimulated with high concentrations of antigen. IgE-sensitized WT and hck−/− BMMCs were stimulated with the indicated concentrations of antigen for 45 minutes (A) or 20 hours (B). Histamine, tumor necrosis factor-α, and IL-6 secreted into culture media were measured. Representative results from 3 experiments are shown. Error bars represent SD.
Microtubule formation is defective in hck−/− mast cells
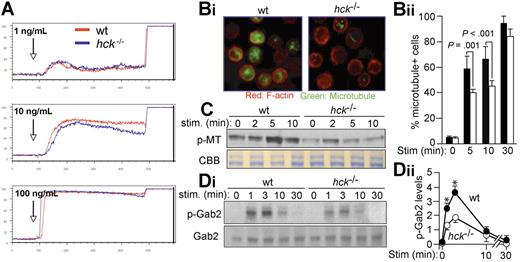
Ca2+ mobilization is required for maximal degranulation in FcϵRI-stimulated cells.35 However, Ca2+ mobilization induced by stimulation of IgE-sensitized cells with various antigen concentrations was comparable between WT and hck−/− cells (Figure 3A), suggesting that the Ca2+-dependent pathway is not affected in hck−/− cells. A recent study revealed 2 steps of FcϵRI-induced degranulation, that is, a Ca2+-independent microtubule-dependent translocation of granules to the plasma membrane and Ca2+-dependent membrane fusion and exocytosis. Consistent with this notion, microtubule formation was defective in hck−/− mast cells, as detected by immunofluorescence staining (Figure 3B-1,B-2). The amount of polymeric tubulin was also reduced in hck−/− cells, as measured by immunoblotting (Figure 3C). Consistent with the role for Gab2 in microtubule formation, Gab2 phosphorylation on Tyr452 was reduced in hck−/− cells (Figure 3D-1,D-2). Therefore, these results indicate that Hck controls degranulation by promoting microtubule formation via Gab2 phosphorylation.
Hck deficiency results in impaired microtubule formation associated with reduced Gab2 phosphorylation. (A) IgE-sensitized WT and hck−/− BMMCs were stimulated with the indicated concentrations of antigen at the indicated points and with 2.5 μg/mL ionomycin 400 seconds later. Ca2+ flux was measured by flow cytometry. Representative results from 3 experiments are shown. (B) IgE-sensitized cells were stimulated with 100 ng/mL DNP23-HSA for 5, 10, and 30 minutes. Immunofluorescence analysis for F-actin (stained by rhodamine-phalloidin) and microtubules (stained by anti-α-tubulin) was performed. Images shown are taken from cells stimulated for 10 minutes (Bi). The percentage of microtubule+ cells is shown in panel Bii. See “Microscopy” for image acquisition information. (C) IgE-sensitized cells were stimulated with 100 ng/mL DNP23-HSA for the indicated periods (minutes). Polymeric tubulin (p-MT) in Triton-insoluble fractions was measured as described in Document S1 (top). An SDS-PAGE gel containing Triton-soluble proteins was stained with Coomassie Brilliant Blue to show that comparable amounts of lysates were used for this assay. (Di) Immunoblot analysis of phospho-Gab2 (Tyr-452) in IgE/antigen-stimulated BMMCs (top panel). The same blot was reprobed with anti-Gab2 (bottom panel). Densitometric analysis was performed (Dii). Values shown in panel Dii represent means from at least 3 independent experiments at each time point. Error bars represent SEM. *Statistically significant differences between WT and hck−/− cells (P < .05 by Student t test).
Hck deficiency results in impaired microtubule formation associated with reduced Gab2 phosphorylation. (A) IgE-sensitized WT and hck−/− BMMCs were stimulated with the indicated concentrations of antigen at the indicated points and with 2.5 μg/mL ionomycin 400 seconds later. Ca2+ flux was measured by flow cytometry. Representative results from 3 experiments are shown. (B) IgE-sensitized cells were stimulated with 100 ng/mL DNP23-HSA for 5, 10, and 30 minutes. Immunofluorescence analysis for F-actin (stained by rhodamine-phalloidin) and microtubules (stained by anti-α-tubulin) was performed. Images shown are taken from cells stimulated for 10 minutes (Bi). The percentage of microtubule+ cells is shown in panel Bii. See “Microscopy” for image acquisition information. (C) IgE-sensitized cells were stimulated with 100 ng/mL DNP23-HSA for the indicated periods (minutes). Polymeric tubulin (p-MT) in Triton-insoluble fractions was measured as described in Document S1 (top). An SDS-PAGE gel containing Triton-soluble proteins was stained with Coomassie Brilliant Blue to show that comparable amounts of lysates were used for this assay. (Di) Immunoblot analysis of phospho-Gab2 (Tyr-452) in IgE/antigen-stimulated BMMCs (top panel). The same blot was reprobed with anti-Gab2 (bottom panel). Densitometric analysis was performed (Dii). Values shown in panel Dii represent means from at least 3 independent experiments at each time point. Error bars represent SEM. *Statistically significant differences between WT and hck−/− cells (P < .05 by Student t test).
Lyn kinase activity is increased in hck−/− mast cells
To investigate the molecular mechanism by which Hck positively regulates FcϵRI-induced activation, we analyzed signaling events in more detail. Immunoblot analysis revealed increased tyrosine phosphorylation of several proteins, including those of 53 and 56 kDa, in hck−/− cells before and after FcϵRI stimulation with IgE plus 100 ng/mL of antigen, compared with WT cells (Figure 4A). Consistent with the possibility that these 53- and 56-kDa proteins contain p53lyn and p56lyn, levels of phosphorylation at Tyr396 in the activation loop were increased in hck−/− cells, whereas phosphorylation at Tyr507 in the C-terminal region of Lyn was not significantly increased. Importantly, the kinase activity of Lyn was increased before, and at early time points of (up to 3 minutes), FcϵRI stimulation (Figures 4B,S2). In contrast, Fyn kinase activity was similar in WT and hck−/− cells (Figure 4B). Of note, Hck deficiency did not affect expression of Lyn and Fyn proteins. Therefore, it seems that Hck negatively regulates Lyn kinase activity constitutively as well as under “high-intensity” FcϵRI stimulation conditions.
Hck deficiency leads to increased Lyn activity and increased phosphorylation of Lyn phosphorylation targets. IgE-sensitized WT and hck−/− cells were stimulated with 100 ng/mL DNP23-HSA for the indicated periods. Cell lysates were either directly analyzed by SDS-PAGE and immunoblotting with the indicated antibodies (A,B,E,G) or first immunoprecipitated (indicated by thick vertical lines on the right of gels) with anti-FcϵRIβ mAb (C) or anti-Cbp/PAG (E,F), and followed by immunoblotting with antiphosphotyrosine mAb (C,E) or anti-Hck antibody (F). (B) Immunoprecipitated SFKs were subjected to in vitro kinase assays. (D) Cell lysates were fractionated into lipid raft and soluble compartments by sucrose density gradient ultracentrifugation. Lipid raft compartments were immunoprecipitated with anti-FcϵRIβ mAb, and followed by immunoblotting with antiphosphotyrosine mAb. Immunoprecipitated antigens were detected by reprobing the blots. Representative results from 2 experiments are shown, except for Lyn and Fyn kinase assays (B), which represent 3 experiments, and phosphotyrosine probing (A), which represent at least 4 experiments.
Hck deficiency leads to increased Lyn activity and increased phosphorylation of Lyn phosphorylation targets. IgE-sensitized WT and hck−/− cells were stimulated with 100 ng/mL DNP23-HSA for the indicated periods. Cell lysates were either directly analyzed by SDS-PAGE and immunoblotting with the indicated antibodies (A,B,E,G) or first immunoprecipitated (indicated by thick vertical lines on the right of gels) with anti-FcϵRIβ mAb (C) or anti-Cbp/PAG (E,F), and followed by immunoblotting with antiphosphotyrosine mAb (C,E) or anti-Hck antibody (F). (B) Immunoprecipitated SFKs were subjected to in vitro kinase assays. (D) Cell lysates were fractionated into lipid raft and soluble compartments by sucrose density gradient ultracentrifugation. Lipid raft compartments were immunoprecipitated with anti-FcϵRIβ mAb, and followed by immunoblotting with antiphosphotyrosine mAb. Immunoprecipitated antigens were detected by reprobing the blots. Representative results from 2 experiments are shown, except for Lyn and Fyn kinase assays (B), which represent 3 experiments, and phosphotyrosine probing (A), which represent at least 4 experiments.
Because Lyn kinase activity was increased before and right after FcϵRI stimulation in hck−/− cells, we predicted that tyrosine phosphorylation of Lyn targets might be increased in hck−/− cells. Lyn phosphorylation targets include FcϵRI β and γ subunits, LAT (linker for activation of T cells),36 Cbp/PAG (a lipid raft-resident protein important for Csk recruitment37,38 ), and NTAL (non–T-cell activation linker)/LAB (linker for activation of B cells).39 Indeed, tyrosine phosphorylation of β and γ subunits was increased in whole cell lysates and lipid raft fractions of hck−/− cells (Figure 4C,D). Tyrosine phosphorylation of LAT (Tyr-191), Cbp/PAG and NTAL/LAB was also increased in hck−/− cells with kinetics similar to those of Lyn kinase activity (Figure 4E and data not shown). Therefore, the increased tyrosine phosphorylation of Lyn substrates in hck−/− cells at early times correlates well with increased Lyn kinase activity.
Lyn deficiency results in increased Fyn activity.11,12 This can be accounted for by Lyn's role in the phosphorylation of Cbp/PAG, which results in the recruitment of Csk to the plasma membrane,37,38 where Fyn is phosphorylated by Csk on its C-terminal negative regulatory residue.40 We next tested whether a similar mechanism, ie, physical association of Hck with Cbp/PAG, might operate for Hck-mediated inhibition of Lyn activity. As shown in Figure 4F, Cbp/PAG associated constitutively with Hck, and this association was increased on FcϵRI stimulation. These results suggest that Hck may phosphorylate Cbp/PAG, leading to inhibition of Lyn activity.
Phosphorylation of SHIP and Dok-2 is upregulated in hck−/− mast cells
Previous studies showed that phosphorylation of SHIP, an important negative regulator in mast-cell activation,41 is Lyn- and β-ITAM-dependent.11,23 Consistent with increased Lyn kinase activity and FcϵRIβ phosphorylation, tyrosine phosphorylation of SHIP was also increased in hck−/− cells on “high-intensity” FcϵRI stimulation, compared with WT cells (Figure 4G). p56dok−2, a p62dok homolog, inhibits IL-2–induced and endothelial growth factor receptor–induced mitogen-activated protein kinase activation.42,43 Not surprisingly, phosphorylation of p56dok−2 was also increased in hck−/− cells (Figure 4G). These results suggest that Hck inhibits negative regulators by downregulating Lyn kinase activity.
Activities of Syk, Btk, p38, JNK, and Akt and IκBα degradation are positively regulated by Hck
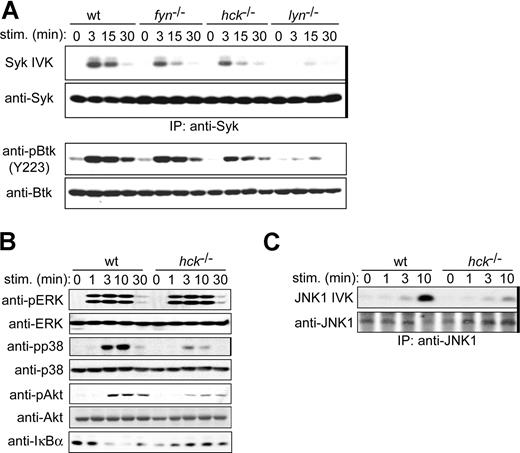
Syk is an essential PTK required for mast-cell activation.21,22 Btk, a Tec family PTK, is also required for FcϵRI-induced cytokine production.44,45 Stimulation with 100 ng/mL of antigen induced strong enzymatic activation of Syk in WT cells, as measured by in vitro kinase assays using immunoprecipitated Syk. As shown previously,10 Syk activity was drastically reduced in lyn−/− cells (Figure 5A). Importantly, Syk activity was more modestly reduced in hck−/− and fyn−/− cells. Phosphorylation of Btk on Tyr223, an autophosphorylation site whose phosphorylation reflects its kinase activity,46 was also reduced in SFK-deficient cells with their rank order of impairment being lyn−/− more than hck−/− more than fyn−/− cells (Figure 5A), consistent with our previous observation that Btk activity is dependent on Syk.47
Hck deficiency results in reduced activities of positive regulatory molecules. IgE-sensitized WT and hck−/− cells were stimulated with 100 ng/mL DNP23-HSA for the indicated periods. (A) Syk was immunoprecipitated (indicated by thick vertical line on the right of gel) from cleared cell lysates and immune complexes subjected to in vitro kinase assays using GST-HS1 as a substrate. Portion of the autoradiogram including GST-HS1 phosphorylation is shown. Cell lysates were directly analyzed by SDS-PAGE and immunoblotting with anti-Syk or anti-phospho-Btk (Tyr223). The pBtk blot was reprobed with anti-Btk antibody. (B) Cell lysates were directly analyzed by SDS-PAGE and immunoblotting with the indicated antibodies. The same blots were reprobed with antibodies that detect antigens irrespective of their phosphorylation status. (C) Immunoprecipitated JNK1 (indicated by thick vertical line on the right of gel) was subjected to in vitro kinase assays. Representative results from 2 experiments are shown.
Hck deficiency results in reduced activities of positive regulatory molecules. IgE-sensitized WT and hck−/− cells were stimulated with 100 ng/mL DNP23-HSA for the indicated periods. (A) Syk was immunoprecipitated (indicated by thick vertical line on the right of gel) from cleared cell lysates and immune complexes subjected to in vitro kinase assays using GST-HS1 as a substrate. Portion of the autoradiogram including GST-HS1 phosphorylation is shown. Cell lysates were directly analyzed by SDS-PAGE and immunoblotting with anti-Syk or anti-phospho-Btk (Tyr223). The pBtk blot was reprobed with anti-Btk antibody. (B) Cell lysates were directly analyzed by SDS-PAGE and immunoblotting with the indicated antibodies. The same blots were reprobed with antibodies that detect antigens irrespective of their phosphorylation status. (C) Immunoprecipitated JNK1 (indicated by thick vertical line on the right of gel) was subjected to in vitro kinase assays. Representative results from 2 experiments are shown.
Downstream of these receptor-proximal PTKs, mitogen-activated protein kinases, Akt, and NF-κB function as intermediary positive regulators for FcϵRI-induced mast-cell activation.45,48,,–51 Stimulation with 100 ng/mL of antigen-induced robust phosphorylation and thus activation of mitogen-activated protein kinases (ERK1, ERK2, and p38) and Akt in WT cells (Figure 5B). In contrast, there was considerably reduced activation of p38 and Akt in hck−/− cells, although phosphorylation of ERK1 and ERK2 was not affected by Hck deficiency. JNK activity was also decreased in hck−/− cells (Figure 5C). Furthermore, FcϵRI stimulation induces IκB kinase-mediated phosphorylation and rapid degradation of IκBα, which binds and masks the NF-κB nuclear localization signal and thus sequesters NF-κB in the cytoplasm.52,53 IκBα degradation was abolished in hck−/− cells, indicating that the NF-κB pathway is positively regulated by Hck (Figure 5B). This result is consistent with our observation that SHIP phosphorylation is increased in hck−/− cells (Figure 4G), combined with observations that SHIP negatively regulates NF-κB and IL-6 production in FcϵRI-stimulated mast cells.51 Taken together, these results indicate that the activities of Syk, Btk, p38, JNK, Akt, and NF-κB are under the control of Hck, which is consistent with reduced cytokine production in hck−/− cells.
“Low-intensity” stimuli uncouple the increased Lyn activity from its negative regulatory function
These signaling studies were performed in the cells stimulated with 100 ng/mL of antigen in “high-intensity” stimulation conditions.23 Compared with these conditions, differences in histamine release and cytokine production induced by stimulation with 1 ng/mL DNP23-HSA (“low-intensity” stimulus) were smaller between WT and hck−/− cells (Figure 2). To examine whether Hck plays any significant roles under “low-intensity” conditions, IgE-sensitized cells were stimulated with 1 ng/mL DNP23-HSA. Tyrosine phosphorylation of cellular proteins including Lyn was generally higher in hck−/− cells unstimulated or stimulated with 1 ng/mL DNP23-HSA compared with WT cells (data not shown), similar to that in hck−/− cells stimulated with 100 ng/mL DNP23-HSA. Importantly, despite the increased Lyn phosphorylation on Tyr396 and kinase activity, phosphorylation of FcϵRIβ and SHIP (ie, Lyn substrates important for negative regulation of mast-cell activation) was not increased in hck−/− cells stimulated with 1 ng/mL DNP23-HSA (Figure 6A). Indeed, FcϵRIβ was not significantly tyrosine-phosphorylated under these conditions in WT or hck−/− cells. Phosphorylation of ERK1/2 and Akt is generally lower and more transient under “low-intensity” stimulation conditions than under “high-intensity” stimulation conditions (Figure S3). Interestingly, LAT-Tyr191, ERK1/2, and p38 were phosphorylated at slightly higher levels and Akt phosphorylation was slightly lower in hck−/− cells than in WT cells (Figure 6B), although it is not clear whether these minor differences in weak signaling translated into biologic consequences. IκBα degradation was not seen in either WT or hck−/− cells.
Stimulation with a low concentration of antigen does not induce phosphorylation of FcϵRIβ or increase SHIP phosphorylation in hck−/− cells despite increased Lyn activity. IgE-sensitized WT and hck−/− cells were stimulated with 1 ng/mL DNP23-HSA for the indicated periods. Cell lysates were directly analyzed by SDS-PAGE and immunoblotting with the indicated phospho-specific antibodies. The same blots were reprobed with antibodies that detect antigens irrespective of their phosphorylation status. (A, third and fourth rows) Immunoprecipitated Lyn was subjected to autophosphorylation assays. Comparable immunoprecipitations were confirmed by immunoblotting. (A, middle) Immunoprecipitated FcϵRIβ was analyzed by immunoblotting with anti-phosphotyrosine mAb and then reprobed with anti-FcϵRIβ mAb. Immunoprecipitations are indicated by thick vertical lines on the right of gels. Representative results from 2 experiments are shown.
Stimulation with a low concentration of antigen does not induce phosphorylation of FcϵRIβ or increase SHIP phosphorylation in hck−/− cells despite increased Lyn activity. IgE-sensitized WT and hck−/− cells were stimulated with 1 ng/mL DNP23-HSA for the indicated periods. Cell lysates were directly analyzed by SDS-PAGE and immunoblotting with the indicated phospho-specific antibodies. The same blots were reprobed with antibodies that detect antigens irrespective of their phosphorylation status. (A, third and fourth rows) Immunoprecipitated Lyn was subjected to autophosphorylation assays. Comparable immunoprecipitations were confirmed by immunoblotting. (A, middle) Immunoprecipitated FcϵRIβ was analyzed by immunoblotting with anti-phosphotyrosine mAb and then reprobed with anti-FcϵRIβ mAb. Immunoprecipitations are indicated by thick vertical lines on the right of gels. Representative results from 2 experiments are shown.
We evaluated the effect of another “low-intensity” stimulus, IgE+anti-IgE, on activation of mast cells. IgE-sensitized WT and hck−/− cells were stimulated with 2 or 20 μg/mL anti-IgE mAb E1B3.23 Similar to cells stimulated with 1 ng/mL DNP23-HSA, cytokine production was similar between WT and hck−/− cells (Figure S4A). Tyrosine phosphorylation of cellular proteins including Lyn was higher in hck−/− cells unstimulated or stimulated with anti-IgE (Figure S4B), similar to that in hck−/− cells stimulated with 1 or 100 ng/mL DNP23-HSA. Despite the increased Lyn phosphorylation on Tyr396, FcϵRIβ was not tyrosine-phosphorylated23 and SHIP phosphorylation was not increased in hck−/− cells (Figure S4B). Furthermore, phosphorylation of ERK1/2, p38, and Akt was either reduced in hck−/− cells or comparable in WT and hck−/− cells (Figure S4C). These results indicate that “low-intensity” FcϵRI stimuli in hck−/− cells uncouple the increased Lyn activity from its ability to exert negative regulation on downstream signaling events (such as FcϵRIβ and SHIP phosphorylation), indicating that Lyn's increased kinase activity per se is not sufficient for its negative regulatory function, but that “high-intensity” stimulus is required.
Positive regulatory roles of Hck can be exerted in Lyn-dependent and Lyn-independent pathways
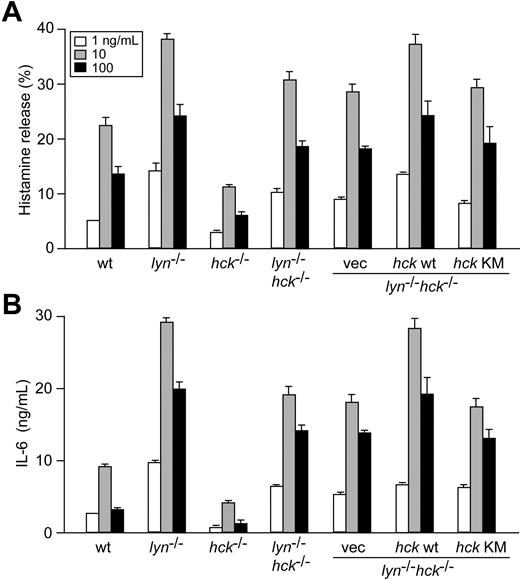
These data suggest that positive signaling roles for Hck are exerted through negative regulation of Lyn activity. To test whether the positive regulatory roles for Hck in mast cells are absolutely Lyn-dependent, mast-cell activation phenotypes were compared between WT, lyn−/−, hck−/−, and lyn−/−hck−/− mice. Comparable expression of FcϵRI and c-Kit on the surface of these mast cells was confirmed by flow cytometry (data not shown). As shown previously,8 histamine release and cytokine production induced by FcϵRI stimulation were increased in lyn−/− cells, but decreased in hck−/− cells, compared with WT cells (Figure 7A,B). Importantly, an intermediate phenotype was noticed in lyn−/−hck−/− cells: both histamine release and cytokine production were higher in lyn−/−hck−/− than in hck−/− cells (and WT cells), but lower than in lyn−/− cells, suggesting that the suppressing activities in hck−/− cells are not totally dependent on Lyn and that Hck positively functions partly independent of Lyn. Moreover, restoration of WT, but not kinase-dead, Hck in lyn−/−hck−/− cells could upregulate histamine release and cytokine production to the levels in lyn−/− cells (Figure 7A,B), demonstrating that the Lyn-independent positive regulatory function of Hck is exerted through its kinase activity. In these hck-transduced cells, Hck expression was very high (approximately 100-fold over its expression level in WT cells, as measured by immunoblotting using predetermined amounts of GST-Hck as a reference). However, Hck overexpression in lyn−/−hck−/− cells simply reversed the defective degranulation/cytokine phenotype to that of lyn−/− cells, indicating that mast cells tolerate overexpression of Hck without their FcϵRI-mediated activation phenotype being affected.
Hck/Lyn doubly deficient mast cells exhibit an intermediate activation phenotype between Hck- or Lyn-deficient cells, and positive and negative regulation via FcϵRI β subunit is exerted by Lyn-mediated phosphorylation of the canonical and noncanonical tyrosine residues. IgE-sensitized mast cells of the indicated genotypes and FcϵRIβ-transduced cells were stimulated with 1 (□), 10 (░), or 100 (■) ng/mL DNP23-HSA for 45 minutes (A) or 20 hours (B). Histamine and IL-6 secreted into culture media were measured. ND indicates not detected. Representative results from 2 independent transduction experiments are shown. Error bars represent SD.
Hck/Lyn doubly deficient mast cells exhibit an intermediate activation phenotype between Hck- or Lyn-deficient cells, and positive and negative regulation via FcϵRI β subunit is exerted by Lyn-mediated phosphorylation of the canonical and noncanonical tyrosine residues. IgE-sensitized mast cells of the indicated genotypes and FcϵRIβ-transduced cells were stimulated with 1 (□), 10 (░), or 100 (■) ng/mL DNP23-HSA for 45 minutes (A) or 20 hours (B). Histamine and IL-6 secreted into culture media were measured. ND indicates not detected. Representative results from 2 independent transduction experiments are shown. Error bars represent SD.
Because a fraction of Lyn and Fyn interacts with the FcϵRI β subunit,3,8 we examined the possibility that Hck might also interact with the FcϵRI β subunit and exert its Lyn-independent function. Robust interactions between Lyn and FcϵRI β subunit were seen by coimmunoprecipitation from 0.2 mg of WT BMMC lysates. However, consistent with a previous report,8 no interaction could be detected between Hck and FcϵRI β subunit using even 100 times more (20 mg of) lysates (data not shown).
The canonical and noncanonical tyrosine residues of FcϵRI β-ITAM, respectively, mediate positive and negative regulatory functions of Lyn and, to a lesser extent, Hck
Previous studies including our own indicate that the negative regulatory role for Lyn is β-ITAM–dependent, and mediated by phosphorylation of the noncanonical β-ITAM tyrosine residue, Y-225.19,20,23 To further dissect the functional relationship between Lyn, Hck, and FcϵRI β subunit, different β-ITAM mutants were introduced retrovirally into lyn−/−FcϵRIβ−/− and hck−/−FcϵRIβ−/− mast cells. Comparable expression of FcϵRI on mast cells expressing WT and mutant FcϵRIβ was confirmed by flow cytometry (data not shown). The lyn−/−FcϵRIβ−/− cells reconstituted with WT FcϵRIβ (designated lyn−/−-YYY cells) largely restored IgE/antigen-induced IL-6 production (Figure 7C), as previously shown.23 The lyn−/−-FFF cells showed a lower, but still substantial, amount of IL-6 production than lyn−/−-YYY cells, indicating that some cytokine production requires neither intact β-ITAM nor Lyn. The lyn−/−-YFY cells induced levels of IL-6 production similar to that in lyn−/−-YYY cells, consistent with the notion that Y-225 is phosphorylated mainly by Lyn. However, lower IL-6 production induced by the lyn−/−-FYF mutant relative to the lyn−/−-FFF mutant suggests that a PTK other than Lyn may also be able to phosphorylate the noncanonical tyrosine residue, Y-225. The lyn−/−-YYF and lyn−/−-FYY mutants induced intermediate levels of IL-6 production, between those induced by lyn−/−-FYF and lyn−/−-FFF, suggesting that both Y-219 and Y-229 residues are important for positive regulation and can be phosphorylated by a PTK other than Lyn. However, YYY expression in hck−/−FcϵRIβ−/− cells restored IL-6 production to a level similar to hck−/− cells (Figure 7D). Expression of YFY induced more IL-6 production than that of YYY, consistent with the role for Lyn in phosphorylation of the negative regulatory noncanonical tyrosine residue. This interpretation was also supported by our observation that IL-6 production in hck−/−-FYF cells was lower than that in hck−/−-FFF cells. IL-6 production was even lower in hck−/−-YYF and hck−/−-FYY cells than in hck−/−-FYF cells, consistent with the notion that phophorylation of Y-219 and Y-229 residues by Hck is important for positive regulation. Therefore, Hck-dependent, Lyn-independent positive regulation also seems to be at least partly FcϵRIβ-dependent. To confirm the ability of Hck to phosphorylate the FcϵRIβ ITAM, we performed in vitro kinase assays using WT and mutant FcϵRIβ peptides. Lyn and Hck immunoprecipitated from BMMC lysates showed a very similar phosphorylating activity toward FcϵRIβ peptides, with the rank order of preference being YYY more than YFF more than FYF more than FFY (Figure S5). These results are in agreement with the preferential phosphorylation of Y-219 of FcϵRIβ molecules expressed in transfected cells.19
Discussion
This study demonstrates positive regulatory functions of Hck in FcϵRI-induced mast-cell activation. These functions are exerted by both Lyn-dependent and Lyn-independent mechanisms. Both mechanisms appear to at least partly involve phosphorylation of the tyrosine residues in the β-ITAM. The Lyn-dependent mechanism is exerted by inhibition of the phosphorylation and catalytic activity of Lyn. These results, together with previous observations that Fyn activity is enhanced in lyn−/− mast cells,11,12 indicate a hierarchical relationship among these SFKs: Hck negatively regulates Lyn and Lyn negatively regulates Fyn.
Here we estimated cellular concentrations of these SFKs in mast cells for the first time. Our measurements confirmed a broadly held assumption that Lyn is the most abundant SFK in mast cells.2,5 Fyn, which is expressed at an approximately 17-fold lower level than p53lyn, was also shown to play a unique role by inducing Gab2 phosphorylation, and thus contributing to degranulation.8 It may appear surprising that p56hck and p59hck, expressed at 30- and 50-fold lower expression levels than p53lyn, play a significant role in mast-cell activation. However, the combined amount of p59hck and p56hck is similar to the amount of p59fyn. Therefore, it may not be so surprising that hck−/− mast cells exhibited defective activation phenotypes, but the results indicate that these SFKs have unique roles in mast cells. This argument is also supported by our observation that 100-fold expression of WT Hck over endogenous levels did not affect activation levels of degranulation or cytokine production. Although concentrations of these kinases at the subcellular locations where they exert their function should be more important than their average cellular concentrations, low expression of Hck hampered further detailed analysis of its subcellular concentrations.
The present study showed that Hck is required for optimal in vitro proliferation of mast cells in response to IL-3 and SCF. However, mast cell numbers in several tissues are comparable between WT and hck−/− mice. In a recent study, lyn−/− mice were shown to have more peritoneal and dermal mast cells than WT mice, and lyn−/− mast cells expand faster in response to IL-3 and SCF.12,54 These contrasting phenotypes might be accounted for by the increased Lyn activity in hck−/− mast cells. However, in another study, bone marrow cells from lyn−/− mice generated similar numbers of mast cells as cells from WT mice did.10 The 2 studies also differed with respect to growth factor withdrawal-induced apoptosis: Hernandez-Hansen et al54 showed less apoptosis in lyn−/− mast cells and the latter showed comparable apoptosis in WT and lyn−/− cells. These differences could be attributable to differences in the genetic background of the mice studied. In this study, hck−/− cells died as fast as WT cells.
The hierarchical relationship among SFKs suggests exquisite mechanisms that mast cells use to fine-tune their activation. Lyn kinase activity is increased in hck−/− cells (this study) and Fyn kinase activity is increased in lyn−/− cells.11,12 c-Src activity is reduced in lyn−/− cells.12 However, Fyn activity is not altered by Hck deficiency and Lyn activity is not altered by Fyn deficiency. Thus, Hck specifically inhibits Lyn activity and Lyn specifically inhibits Fyn activity in mast cells. SFK activity is positively regulated by phosphorylation of the tyrosine residue (Tyr396 in Lyn) in the activation loop,55,56 whereas phosphorylation of the C-terminal tyrosine residue (Tyr507 in Lyn) by Csk inhibits its kinase activity.40 Csk is recruited to the plasma membrane by tyrosine-phosphorylated Cbp/PAG via interactions between Csk's SH2 domain and phosphorylated Tyr-314 of Cbp/PAG.37,38 Consistent with previous studies that Cbp/PAG is phosphorylated by Lyn,12 tyrosine phosphorylation of Cbp/PAG is increased in hck−/− mast cells in which Lyn activity is increased. Lyn-mediated Cbp/PAG phosphorylation can account for Lyn-dependent Fyn inhibition. A similar mechanism might operate for Hck-mediated Lyn inhibition, because Hck is physically associated with Cbp/PAG (Figure 4F). However, this scenario cannot explain why Fyn activity is not increased in hck−/− cells and c-Src activity is not increased in lyn−/− cells. It is not clear whether Cbp/PAG phosphorylation affects each SFK with equal potency, although localization of each SFK relative to that of Cbp/PAG may be important for their activity. Another potential, nonmutually exclusive mechanism for hierarchical regulation among SFKs can be through regulation of protein-tyrosine phosphatases that dephosphorylate critical tyrosine residues of SFKs. This counteracting response is induced in response to an activating mutation in Hck, HckY499F.57 This response might be dampened in hck−/− cells, leading to the enhanced Lyn activity. Phosphorylation of Lyn on Tyr396 is increased in hck−/− cells. Future investigation into these regulations of Hck versus other SFKs will be necessary for our better understanding of the initial activation mechanisms of mast cells.
Transduction of FcϵRIβ mutants in lyn−/−FcϵRIβ−/− cells confirmed that the canonical tyrosine residues of β-ITAM are involved in Lyn-dependent positive regulation of mast-cell activation, whereas the noncanonical tyrosine residue is involved in Lyn-dependent negative regulation. Similar experiments with hck−/−FcϵRIβ−/− cells not only supported Lyn's roles in positive and negative regulation through phosphorylation of β-ITAM tyrosine residues but also suggested Hck's role in positive regulation by phosphorylating the canonical tyrosine residues. Therefore, the Lyn-independent positive regulatory function of Hck also appears to involve, at least in part, β-ITAM phosphorylation (Figure S6). Interestingly, the noncanonical tyrosine Tyr-225 can be phosphorylated by a PTK other than Lyn (albeit to a lesser extent), because IL-6 production was lower in lyn−/−-FYF than in lyn−/−-FFF cells. To gain a better understanding of these key regulatory mechanisms in mast-cell activation, further study on other SFKs is warranted.
Parravicini et al8 suggested that FcϵRI can use an alternative activation pathway for mast-cell degranulation that involves Fyn-mediated Gab2 phosphorylation and subsequent phosphatidylinositol 3-kinase activation. Recently, this Fyn/Gab2 pathway was shown to be required for microtubule formation and consequent translocation of granules to the plasma membrane.9 Unlike Parravicini et al, Yu et al58 suggested that Syk is the kinase that phosphorylates Gab2. Importantly, Hck deficiency results in defective Gab2-Tyr452 phosphorylation and microtubule formation, leading to reduced degranulation, despite normal levels of Fyn kinase activity. Our study also showed that Syk activity was reduced in hck−/− cells. Irrespective of which PTK is responsible for Gab2 phosphorylation, these results indicate that both Fyn and Hck are required for Gab2-dependent degranulation.
Topographical studies point to the critical importance of locations of FcϵRI and signaling molecules for their proper functioning.59,,,–63 They support the notion that there are functional and nonfunctional pools of signaling molecules: it is tempting to speculate that a pool of Lyn molecules, probably those prebound to FcϵRIβ, can phosphorylate the canonical β-ITAM residues and γ-ITAM to initiate FcϵRI signaling; another pool of Lyn molecules, which have a configuration relative to FcϵRIβ different from the activating Lyn pool, phosphorylates the noncanonical tyrosine residue to trigger the negative regulatory signal. Similar to FcϵRIβ-bound Lyn, FcϵRIβ-bound Fyn as well as receptor-proximal Hck molecules might belong to an activating pool that can phosphorylate the canonical β-ITAM residues (and γ-ITAM). However, it is not clear whether Hck plays a negative regulatory role, whereas Fyn deficiency results in increased IL-13 production.64
In conclusion, Hck plays a positive regulatory role in FcϵRI-stimulated mast-cell activation probably by phosphorylating the canonical tyrosine residues in β-ITAM and suppressing Lyn kinase activity. Together with previous studies showing Lyn-mediated Fyn inhibition, these 3 SFKs exhibit a hierarchical relationship, ie, Hck inhibits Lyn and Lyn inhibits Fyn. This hierarchical relationship seems critical in fine-tuning mast-cell activation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Mari Maeda-Yamamoto for histamine measurements; Drs Daniel H. Conrad, Yasuko Furumoto, Juan Rivera, and Alexander Y. Tsygankov for providing reagents; and Dr Michael Poderycki for critical reading of the manuscript.
This work was supported in part by National Institutes of Health grants AI-38348 and AI-50209 (T.K.). This article is publication no. 866 from the La Jolla Institute for Allergy and Immunology.
National Institutes of Health
Authorship
Contribution: H.H., J.K., and W.X. performed experiments. V.H., C.R., and C.A.L. provided crucial reagents. Y.K., W.X., and T.K. designed experiments. T.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshiaki Kawakami, Department of Cell Biology, La Jolla Institute for Allergy and Immunology, 9420 Athena Circle, La Jolla, CA 92037; e-mail:toshi@liai.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal