Circulating monocytes can differentiate into dendritic cells (moDCs), which are potent inducers of adaptive immune responses. Previous reports show that granulocyte macrophage–colony-stimulating factor (GM-CSF) and interleukin-4 induce monocyte differentiation into moDCs in vitro, but little is known about the physiological requirements that initiate moDC differentiation in vivo. Here we show that a unique natural killer (NK) cell subset (CD3−CD56bright) that accumulates in lymph nodes and chronically inflamed tissues triggers CD14+ monocytes to differentiate into potent T-helper-1 (TH1) promoting DC. This process requires direct contact of monocytes with NK cells and is mediated by GM-CSF and CD154 derived from NK cells. It is noteworthy that synovial fluid (SF) from patients with rheumatoid arthritis (RA) and psoriatic arthritis (PsA), but not osteoarthritis (OA), induces monocytes to differentiate into DC. However, this process occurs only in the presence of NK cells. We propose that NK cells play a role in the maintenance of TH1-mediated inflammatory diseases such as RA by providing a local milieu for monocytes to differentiate into DC.
Introduction
Dendritic cells (DCs) are extremely potent antigen-presenting cells (APCs), capable of initiating primary T-cell responses by presenting antigenic peptides in association with major histocompatibility complex (MHC) class I and II molecules on the cell surface (reviewed in Banchereau and Steinman1 ). Circulating DCs are rare, necessitating the use of in vitro cultivation protocols to generate sufficient DCs from bone marrow or monocytes for in vitro studies or therapeutic use.2,,–5 One such protocol, culturing peripheral blood (PB) derived CD14+ monocytes in the presence of exogenous granulocyte macrophage–colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) for 7-10 days,2 has become the standard for production of human monocyte-derived DCs (moDCs). These immature moDC can be further matured by exposing the cells to toll-like receptor agonists or molecules such as tumor necrosis factor α (TNFα), interferon-γ (IFNγ), and CD40 ligand (CD154).1,6 Although these in vitro methods have been shown to generate moDCs that can induce immune responses in vitro and in vivo, it is unknown whether such cells correspond to any DC subset present in vivo.
DCs are believed to contribute to the pathophysiology of inflammatory diseases such as rheumatoid arthritis (RA), which is characterized by chronically inflamed joints and other characteristic findings.7,–9 A variety of leukocytes accumulate in the RA synovium and synovial fluid, including large numbers of monocytes derived from the circulation.10 DCs are also present in these tissues, although the physiologic processes that guide their differentiation from monocytes are not known.
Like monocytes, circulating natural killer (NK) cells are recruited to inflamed tissues, including the RA joint,11,,,–15 and they coexist in various lymphoid organs with monocytes and DCs.16,,,,–21 NK cells were initially defined on the basis of their cytotoxic activity22 ; this activity, together with their production of both proinflammatory and anti-inflammatory cytokines, is believed to be important in the defense against infectious agents, especially viruses (reviewed in Lanier23 ). Other studies have suggested that NK cells can regulate myelopoiesis24,–26 and induce maturation of GM-CSF and IL-4 derived moDCs in vitro,27,–29 but whether NK cells affect early stages of monocyte differentiation into moDCs is not known. On this basis, and given the colocalization of NK cells with monocytes and DCs in the inflamed RA joint, we reasoned that NK cells might play a role in the differentiation of monocytes into moDCs.
Materials and methods
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) from healthy volunteer blood donations at the Stanford Blood Center and synovial fluid mononuclear cells (SFMCs) from patients with immune-mediated arthritis were isolated by Ficoll density gradient centrifugation. CD14+ monocytes and CD56+ NK cells were purified from PBMCs or SFMCs using anti-CD14 and anti-CD56–coated microbeads (Miltenyi Biotec, Auburn, CA), respectively, followed by 2 rounds of positive selection using autoMACS (Miltenyi Biotec). Enriched CD56+ cells were subsequently depleted of CD3+ cells using anti-CD3–coated Dynal Dynabeads (Invitrogen, Carlsbad, CA). Where indicated CD56bright and CD56dim PB NK-cell populations were obtained by sorting on a FACSVantage (BD Biosciences, San Jose, CA). The purities of freshly isolated CD14+ monocytes and CD3−CD56+ NK cells was always more than 95% as analyzed by flow cytometry. Isolated pure CD3−CD56+ NK-cell lines and clones were cultured as described previously.30
Monocytes were cultured in 24-well plates (Corning, Corning, NY) at 5 × 105 cells/well. For cocultures, 5 × 104 NK cells/well were used at a monocyte to NK-cell ratio of 10:1, which resulted in maximal phenotypic changes in monocytes based on CD14 and DC-SIGN expression in titration experiments (data not shown). The cells were cultured for 6 days, unless otherwise stated, in Iscove's modified Dulbecco medium (Invitrogen) supplemented with 10% fetal calf serum, 2% human serum, 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10 ng/mL IL-15 (Peprotech, Rocky Hill, NJ) where indicated. AIM-V (Invitrogen) was used to verify that the presence of serum did not affect the outcome of the coculture experiments (data not shown). DCGM/IL4 were generated from CD14+ monocytes by feeding the cultures every second day with medium supplemented with 50 ng/mL hGM-CSF and 20 ng/mL hIL-4 (Peprotech). Transwell experiments were performed using 24-well transwell inserts (Corning) with a diameter of 6.5 mm and a pore size of 0.4 μm, and NK cells were cultured in the insert. To block endogenous GM-CSF, IFNγ, and TNFα, neutralizing anti–human GM-CSF mAb (clone GM4.1.9; Calbiochem; San Diego, CA), soluble recombinant human IFNγR1 (R&D Systems, Minneapolis, MN), and soluble recombinant human TNFα receptor (sTNFRI; Peprotech) were used, whereas surface CD154 was blocked with anti-CD154 mAb (clone 24-31; BioLegend, San Diego, CA). All blocking mAbs and soluble receptors were used at 10 μg/mL.
SF from patients with RA, psoriatic arthritis (PsA), and osteoarthritis (OA) was aspirated from the knee joint and 10% cell-free SF was used in cocultures between CD14+ monocytes and autologous NK cells of healthy persons. Ethical approvals for all studies have been obtained by institutional review boards at Karolinska Institute and Stanford University. Informed consent for this study was obtained in accordance with the Declaration of Helsinki.
Antibodies, enzyme-linked immunosorbent assay, and immunofluorescence
Cells were harvested using 5 mM EDTA in phosphate-buffered saline (PBS) and preincubated with FcR-blocking reagent according to the manufacturer's instructions (Miltenyi). Cell staining was performed using fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin (APC) conjugated mouse monoclonal antibodies (mAbs) against CD3, CD4, CD8, CD14, CD56, CD69, CD205 (DEC205), CD209 (DC-SIGN), CD80, CD86, CD40, CD83, CD154, HLA-DR, CD11c, CD33, CD13, CD45RO, CD45RA, CD123, CD1a, CD1b, CCR7, and mouse IgG isotype-matched controls (all from BD Biosciences), CD14 (Dako North America, Carpinteria, CA), ILT3 (R&D Systems), and BDCA2 (Miltenyi Biotec) according to standard procedures. Cells were analyzed on a FACSCalibur (BD Biosciences) and NK cells were excluded based on combined gating of CD56− cells and their forward scatter (FSC):side scatter (SSC) profile. To stain NK cells for CD154 and GM-CSF, 105 cells derived from purified CD3−CD56+ NK-cell lines grown in IL-15 were washed in PBS and incubated with 5 μL FcR-blocking reagent (Miltenyi) together with APC-conjugated mouse anti–human CD154 and PE-conjugated rat anti–human GM-CSF (BD Biosciences) or with corresponding isotype control antibodies at 37°C for 20 minutes followed by 2× washing with 3 mL PBS. ELISA measured soluble GM-CSF in cell-free culture supernatants according to the manufacturer's instructions (R&D Systems).
The mAb 15A11 (mouse IgM) secreting hybridoma clone was derived from standard methods by fusing mouse spleen cells with sp2/0 cells of mice that have been previously immunized with a HLA class I-transfected human LCL cell line (721.221). A hybridoma clone was selected for binding to human CD56+CD3− cells by flow cytometry. The molecule recognized by 15A11 is present on freshly isolated NK cells, and the cell-surface staining is gradually lost upon prolonged in vitro culture. Preliminary studies to further characterize the 15A11-ligand have been unsuccessful.
In some assays CD14+ cells were cultured on glass coverslips in the presence of GM-CSF/IL-4, or in the presence of autologous IL-15-activated NK cells. LPS (1 μg/mL, Sigma) was added on day 5 to induce DC maturation. After 6 days, the cells were fixed for 15 minutes in PBS containing 4% paraformaldehyde, blocked for 15 minutes with PBS containing 0.5% gelatin and 0.25% bovine serum albumin, and incubated with 5 μg/mL FITC-conjugated anti-human leukocyte antigen (HLA)-DR mAb (BD Biosciences) in PBS. After washing, the cells were permeabilized for 20 minutes in Cytofix/Cytoperm solution (BD Biosciences), washed with Perm/Wash buffer (BD Biosciences), followed by staining using 5 μg/mL PE-conjugated anti–DC-LAMP (Beckman Coulter, Fullerton, CA) diluted in Perm/Wash buffer. Mouse IgG2a-FITC and IgG1-PE controls were purchased from BD Biosciences. After washing, coverslips were mounted on microscope slides and evaluated by Leica DMIRB fluorescence microscopy and images were acquired using a digital ORCA-ER camera (Hamamatsu, Bridgewater, NJ). Appropriate filters for immunofluorescence analysis of labeled cells were used, and images were acquired using Openlab software (Improvision) and subsequently imported into Photoshop version 7.0 (Adobe Systems, Mountain View, CA).
For intracellular cytokine staining, brefeldin A (at 10 μg/mL; Sigma) was added to the cultures 6 hours before harvesting. After surface staining with CD3-FITC mAb (BD Biosciences) and washing, the cells were fixed and permeabilized by Cytofix/Cytoperm for 30 minutes at + 4°C, washed twice with Perm/Wash buffer, and stained with PE-conjugated anticytokine mAbs (IFNγ, IL-4) or isotype-matched control mAbs (all reagents purchased from BD Biosciences). Cells were analyzed by flow cytometry.
Immunohistology
For double staining of human NK cells and monocytes, slides with frozen sections of RA tissue where incubated with 15A11 mAb and mouse anti–human CD14 mAb (Dako North America) following standard protocols. In brief, endogenous peroxidase activity was blocked with 1% H2O2 in methanol, and slides were then incubated with 2% normal goat serum in 0.1 M Tris-buffer before adding 15A11 mAb. Binding was detected by goat anti–mouse secondary polyclonal ab (Dako North America) and tertiary alkaline phosphatase complex (Dako North America) using alkaline phosphatase substrate kit III (Vector Laboratories, Burlingame, CA). After the first staining, slides were saturated with normal goat serum before adding CD14 or DC-LAMP mAb. Binding was detected by peroxidase-coupled histofine (Nichirei Biosciences, Tokyo, Japan) using AEC peroxidase substrate kit (Vector Laboratories).
Antigen uptake
To analyze endocytic activity, DCs were cultured at 37°C with 25 μg/mL DQ-ovalbumin (DQ-OVA, Molecular Probes). After overnight culture, the cells were washed twice with PBS and stained for cell surface markers for analysis by flow cytometry. To assess phagocytic activity, we used carboxylate-conjugated yellow-green latex beads 1 μm in diameter (Invitrogen). Cells (105 cells/well) were incubated with beads in flat-bottomed 96-well plates (Costar; Corning Life Sciences, Acton, MA) at a ratio of 5:1 (bead/cell) for 1 hour at 37°C, thereafter washed extensively with PBS and subsequently stained for surface markers. The percentage of cells phagocytosing the beads was determined by flow cytometry.
Mixed lymphocyte reaction
CD4+ T cells were enriched from PBMC by negative depletion using a cocktail of biotin-conjugated antibodies against CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCRγδ, and CD235a followed by anti-biotin–coated microbeads (Miltenyi Biotec). The resultant cells had a purity of more than 95% CD3+ CD4+ as analyzed by flow cytometry. DCGM/IL4 were generated from CD14+ monocytes by feeding the cultures every second day with medium supplemented with 50 ng/mL GM-CSF and 20 ng/mL IL-4 (Peprotech). DCNK were generated as above and were isolated by depleting NK cells using mouse anti-CD56 mAb and goat anti–mouse IgG dynabeads (Dynal) on day 6. DCGM/IL4 and DCNK were subsequently stimulated with TNFα (10 ng/mL), LPS (10 μg/mL) or left in medium alone overnight. The DCs were thereafter harvested, washed 3 times in PBS, irradiated (3000 rad) before incubation with naive CD4+ T cells (105/well) for 7 days in 96-well round-bottom microplates (Costar) at various DC:T cell ratios as indicated. The cultures were pulsed with 1 μCi/well of 3H-thymidine for the last 18 hours of culture. The cells were harvested by Harvester 400 (Tomtec) and radioactivity was measured by 1450 MicroBeta counter (LKB Wallac). The results represent mean counts per minute (± SD) of triplicate cultures.
Results
Monocytes in coculture with activated NK cells differentiated into cells with a DC-like morphology and phenotype
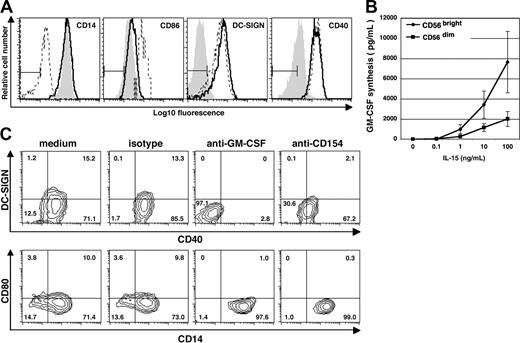
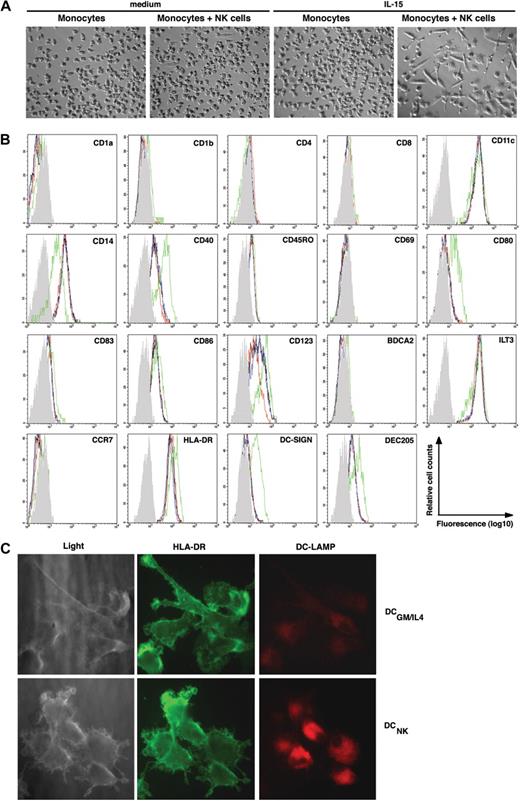
To determine whether NK cells affect monocyte differentiation, freshly isolated CD14+ monocytes were cocultured with autologous NK cells at a ratio of 10:1 in the presence or absence of exogenous IL-15, a cytokine known to activate NK cells.31 Over 3 to 5 days, a large fraction of the monocytes acquired morphologic changes typically associated with DCs, such as elongated cell bodies and a stellate-like morphology (Figure 1A). A smaller number of such cells was also seen in NK cell–monocyte cocultures in the absence of IL-15 (Figure 1A). In contrast, the morphology of monocytes cultured in medium alone, or in medium supplemented with IL-15, did not change (Figure 1A). In the presence of NK cells, the majority of monocytes down-regulated CD14 (Figure 1B shows a representative result), and concomitantly up-regulated CD40, CD80, CD86, DC-SIGN, and DEC205 (Figure 1B and data not shown), molecules characteristic of moDCs. Although the cells up-regulated CD123 (ie, IL-3 receptor α chain), which is typically associated with the plasmacytoid DC (pDC) lineage, they lacked detectable levels of BDCA-2 (Figure 1B), a molecule expressed exclusively on pDC.32 Similar to circulating immature moDCs, the DCs generated in these cultures remained negative for the Langerhans cell marker CD1a but a minor subset acquired CD1b (Figure 1B and data not shown). Monocytes cultured in medium alone, or in the presence of IL-15, remained CD14+ and, except for up-regulated CD40 levels observed upon IL-15 stimulation in some donors (data not shown), no other apparent morphologic or phenotypic change was observed (Figure 1A,B).
IL-15–stimulated NK cells induce monocyte differentiation into DCNK in vitro. (A) Phase contrast microscopy (original magnification, ×100; 10×/0.30 phase objective lens) pictures showing (left to right): monocytes cultured for 6 days in medium alone, monocytes cocultured with NK cells in medium alone, monocytes cultured in the presence of IL-15, or monocytes cultured with NK cells in the presence of IL-15, as indicated on the top of each picture. Images were acquired at 25°C using the equipment described in “Antibodies, enzyme-linked immunosorbent assay, and immunofluorescence,” are representative of 5 separate experiments. (B) Histograms show the phenotype of monocytes after culture in medium alone (black line), or in the presence of IL-15 (blue line), and monocytes that have been cocultured with NK cells in medium alone (red line), or in the presence of IL-15 (green line). The gray histogram represents the isotype control staining of monocytes that have been cocultured with NK cells in the presence of IL-15 (ie DCNK). The data are representative of 3 separate experiments. (C) Light microscopy (left panels) of DCs generated from monocytes upon NK-cell coculture (DCNK bottom) or DCs generated from monocytes in the presence of GM-CSF and IL-4 (DCGM/IL4 top). The DCs were surface stained with anti–HLA-DR (FITC, middle panels) and intracellular DC-LAMP (PE, right panels) and imaged by fluorescence microscopy (original magnification, ×630; 63×/1.40-0.60 oil-immersion objective lens). The results shown are representative of 2 separate experiments.
IL-15–stimulated NK cells induce monocyte differentiation into DCNK in vitro. (A) Phase contrast microscopy (original magnification, ×100; 10×/0.30 phase objective lens) pictures showing (left to right): monocytes cultured for 6 days in medium alone, monocytes cocultured with NK cells in medium alone, monocytes cultured in the presence of IL-15, or monocytes cultured with NK cells in the presence of IL-15, as indicated on the top of each picture. Images were acquired at 25°C using the equipment described in “Antibodies, enzyme-linked immunosorbent assay, and immunofluorescence,” are representative of 5 separate experiments. (B) Histograms show the phenotype of monocytes after culture in medium alone (black line), or in the presence of IL-15 (blue line), and monocytes that have been cocultured with NK cells in medium alone (red line), or in the presence of IL-15 (green line). The gray histogram represents the isotype control staining of monocytes that have been cocultured with NK cells in the presence of IL-15 (ie DCNK). The data are representative of 3 separate experiments. (C) Light microscopy (left panels) of DCs generated from monocytes upon NK-cell coculture (DCNK bottom) or DCs generated from monocytes in the presence of GM-CSF and IL-4 (DCGM/IL4 top). The DCs were surface stained with anti–HLA-DR (FITC, middle panels) and intracellular DC-LAMP (PE, right panels) and imaged by fluorescence microscopy (original magnification, ×630; 63×/1.40-0.60 oil-immersion objective lens). The results shown are representative of 2 separate experiments.
Significant differences in surface phenotype were observed between DCs generated in NK-cell cocultures (here termed DCNK) and DCs generated in medium containing exogenous GM-CSF and IL-4 (termed DCGM/IL4). For example, CD1a, CD1b, DC-SIGN, and CD86 were consistently expressed at higher levels on DCGM/IL4, whereas CD40 expression was consistently higher on DCNK (data not shown). Similar to what has been described for monocytes and immature DCGM/IL4 the DCNK retained expression of ILT3 and remained negative for CCR7 (Figure 1B).33,34 Moreover, the expression of intracellular DC-LAMP, which is found mainly on mature DC in vivo,35 was consistently expressed at higher levels on mature DCNK compared with DCGM/IL4 (Figure 1C).
The capacity of CD56bright PB NK cells to induce monocyte differentiation was more potent than that of CD56dim PB NK cells
Human NK cells in PB can be divided into CD56bright and CD56dim subsets.36,37 In contrast to the CD56dim NK cells, which predominate in PB, the CD56bright subset expresses high levels of lymph-node homing molecules CD62L and CCR711 and is the main NK subset found in the T-cell areas of lymph nodes17,19 and sites of chronic inflammation.12,14,15 Based on their cytokine-producing capacity and similar phenotype to the NK cells found in lymph nodes,17,19,37 we reasoned that CD56bright PB NK cells might be particularly efficient in triggering monocyte differentiation into DCNK. To this end, CD14+ monocytes were cocultured with sorted autologous CD56dim or CD56bright NK cells in the absence (data not shown) or presence of IL-15. As shown in Figure 2, the CD56bright NK cells induced autologous monocytes to differentiate into DCNK, as judged by their capacity to down-modulate CD14 expression and up-regulate DC-SIGN and CD40; in agreement with our previous data, IL-15 was necessary for this process. By contrast, in the presence of CD56dim NK cells, a less pronounced up-regulation of CD40 and DC-SIGN was observed, and CD14 expression levels were maintained. Based on these results, we concluded that both CD56dim and CD56bright PB NK cells could induce phenotypic changes in monocytes and that the CD56bright PB NK-cell subset is more potent than the CD56dim subset in its capacity to induce DCNK differentiation.
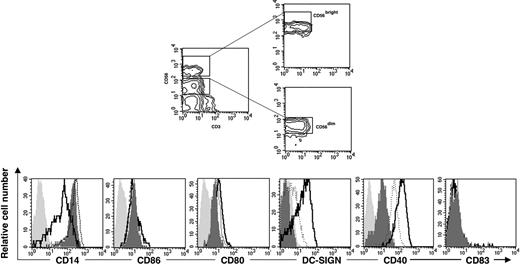
The CD56bright NK-cell subset in PB is mainly responsible for mediating monocyte differentiation into DCs. The CD56bright and CD56dim PB-NK-cell subsets were sorted as shown in the contour graphs (top panels). The purified NK-cell subsets were thereafter cocultured with CD14+ monocytes in the presence of IL-15 at a 10:1 ratio (monocyte/NK cell) for 6 days. Cell surface expression of CD14, CD86, CD80, DC-SIGN, CD40, and CD83 was analyzed after monocyte culture in the presence of IL-15 alone (filled dark gray histogram) or after coculture with CD56bright NK cells (solid line) or with CD56dim NK cells (dotted line). The filled light gray histograms show the isotype control stainings. Data are representative of 3 separate experiments.
The CD56bright NK-cell subset in PB is mainly responsible for mediating monocyte differentiation into DCs. The CD56bright and CD56dim PB-NK-cell subsets were sorted as shown in the contour graphs (top panels). The purified NK-cell subsets were thereafter cocultured with CD14+ monocytes in the presence of IL-15 at a 10:1 ratio (monocyte/NK cell) for 6 days. Cell surface expression of CD14, CD86, CD80, DC-SIGN, CD40, and CD83 was analyzed after monocyte culture in the presence of IL-15 alone (filled dark gray histogram) or after coculture with CD56bright NK cells (solid line) or with CD56dim NK cells (dotted line). The filled light gray histograms show the isotype control stainings. Data are representative of 3 separate experiments.
DCNK efficiently took up antigen by endocytosis, lost the ability to phagocytose, and induced CD4+ T-cell activation and polarization to TH1 cells
As monocytes differentiate into DCs, they maintain endocytic activity but lose their phagocytic capacity and concomitantly acquire the capacity to stimulate naive T cells.1 To analyze endocytic activity, we used a self-quenched conjugate of ovalbumin that exhibits bright green fluorescence only upon endocytosis and intracellular proteolysis. As shown in Figure 3A, DCNK exhibited potent endocytic capacity, as reflected by their uniformly high fluorescence intensity. In fact, ovalbumin uptake by these cells was increased compared with that of monocytes alone or DCs generated in GM-CSF and IL-4. To assess whether DCNK lose their phagocytic capacity during differentiation, we analyzed their capacity to take up large FITC-conjugated latex beads. Monocytes cultured in medium alone, or in the presence of IL-15, readily phagocytosed the beads (Figure 3B). In contrast, and similar to DCGM/IL4, DCNK lost phagocytic activity. It is noteworthy that monocytes cocultured with autologous NK cells in medium alone showed reduced capacity to ingest large beads, indicating that NK cells can induce functional changes in monocytes even in the absence of exogenous IL-15.
DCNK take up antigen by endocytosis, lose phagocytic capacity, and exhibit potent T-cell stimulatory ability. (A) Antigen uptake by endocytosis as assessed by adding DQ-OVA to monocytes cultured in medium alone (filled gray histogram) or in the presence of GM-CSF and IL-4 (- - -), or monocytes cocultured with an NK-cell clone (–). Cells were incubated with DQ-OVA overnight and subsequently analyzed by flow cytometry. A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles. The histogram of NK cells alone (using a combined gate of CD56+ and FSC:SSC) is shown to indicate the fluorescence intensity of nonendocytic NK cells (· · ·). (B) Phagocytic activity, as measured by uptake of FITC-conjugated latex beads of monocytes cultured in medium alone, with IL-15, with NK cells in medium alone (monocytes NKs cells), NK cells and IL-15 (DCNK), or GM-CSF and IL-4 (DCGM/IL4). A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles. Percentages indicate phagocyte activity. Results are representative of 2 separate experiments. (C) DCNK induce potent proliferation of naive allogeneic CD4+ T cells. DCGM/IL4 (circles) and DCNK (triangles) were isolated on day 6 and stimulated with TNFα (gray, 10 ng/mL), LPS (black, 1 μg/mL), or left in medium alone overnight (white). The DC were thereafter irradiated (3000 rad) and incubated at graded doses with naive CD4+ T cells as indicated. After 7 days, the cells were pulsed with [3H]thymidine and proliferation is expressed in counts per minute (cpm) × 103 (mean ± SD of triplicate cultures). (D) DCNK or DCGM/IL4 were stimulated overnight with either 10 ng/mL TNFα or medium alone before coculture with T cells (1:1 ratio) as indicated. Assessment of intracellular IFNγ (top) and IL-4 (bottom) was done using PE-conjugated anti-IFNγ and anti–IL-4 mAbs after a 72-hour culture, and the percentages of cytokine positive cells are indicated in each dot plot. IFNγ or IL-4 produced by T cells without DC stimulation, or by monocyte stimulation, was less than 0.1%. The vertical line is set to indicate the background of the isotype control staining. Results are representative of 3 separate experiments. (E) Phenotypic maturation of DCNK (top panels) and DCGM/IL4 (bottom panels). Parallel cultures of DCNK and DCGM/IL4 were harvested on day 6 and stimulated with 10 ng/mL TNFα, or kept in medium alone, for an additional 48 hours. Cell surface expression of CD40, CD80, CD83, and CD86 on TNFα-matured DC (–) is compared with immature DC (- - -). Gray histograms show staining with isotype control antibody.
DCNK take up antigen by endocytosis, lose phagocytic capacity, and exhibit potent T-cell stimulatory ability. (A) Antigen uptake by endocytosis as assessed by adding DQ-OVA to monocytes cultured in medium alone (filled gray histogram) or in the presence of GM-CSF and IL-4 (- - -), or monocytes cocultured with an NK-cell clone (–). Cells were incubated with DQ-OVA overnight and subsequently analyzed by flow cytometry. A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles. The histogram of NK cells alone (using a combined gate of CD56+ and FSC:SSC) is shown to indicate the fluorescence intensity of nonendocytic NK cells (· · ·). (B) Phagocytic activity, as measured by uptake of FITC-conjugated latex beads of monocytes cultured in medium alone, with IL-15, with NK cells in medium alone (monocytes NKs cells), NK cells and IL-15 (DCNK), or GM-CSF and IL-4 (DCGM/IL4). A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles. Percentages indicate phagocyte activity. Results are representative of 2 separate experiments. (C) DCNK induce potent proliferation of naive allogeneic CD4+ T cells. DCGM/IL4 (circles) and DCNK (triangles) were isolated on day 6 and stimulated with TNFα (gray, 10 ng/mL), LPS (black, 1 μg/mL), or left in medium alone overnight (white). The DC were thereafter irradiated (3000 rad) and incubated at graded doses with naive CD4+ T cells as indicated. After 7 days, the cells were pulsed with [3H]thymidine and proliferation is expressed in counts per minute (cpm) × 103 (mean ± SD of triplicate cultures). (D) DCNK or DCGM/IL4 were stimulated overnight with either 10 ng/mL TNFα or medium alone before coculture with T cells (1:1 ratio) as indicated. Assessment of intracellular IFNγ (top) and IL-4 (bottom) was done using PE-conjugated anti-IFNγ and anti–IL-4 mAbs after a 72-hour culture, and the percentages of cytokine positive cells are indicated in each dot plot. IFNγ or IL-4 produced by T cells without DC stimulation, or by monocyte stimulation, was less than 0.1%. The vertical line is set to indicate the background of the isotype control staining. Results are representative of 3 separate experiments. (E) Phenotypic maturation of DCNK (top panels) and DCGM/IL4 (bottom panels). Parallel cultures of DCNK and DCGM/IL4 were harvested on day 6 and stimulated with 10 ng/mL TNFα, or kept in medium alone, for an additional 48 hours. Cell surface expression of CD40, CD80, CD83, and CD86 on TNFα-matured DC (–) is compared with immature DC (- - -). Gray histograms show staining with isotype control antibody.
The capacity to stimulate allogeneic T cells is a defining property of DC. As shown in Figure 3C, DCNK stimulated with LPS or TNFα induced proliferation of allogeneic CD4+ T cells in a conventional mixed lymphocyte reaction. In addition, CD4+ T cells stimulated by DCNK consistently produced higher levels of IFNγ compared with CD4+ T cells stimulated with DCGM/IL4. Few T cells produced IL-4 when stimulated with DCNK (Figure 3D). Monocytes cultured with IL-15 or in medium alone did not induce a detectable IFNγ response by CD4+ T cells (data not shown). Consistent with the potent stimulatory effect of DCNK on CD4+ T-cell responses, DCNK similar to DCGM/IL4 expressed increased cell surface levels of CD40, CD80, and CD83 (Figure 3E) upon maturation. In contrast to DCGM/IL4, mature DCNK displayed decreased CD86 levels upon maturation in the presence of TNFα.
DCNK differentiation was dependent on GM-CSF and CD154
To determine whether direct contact between NK cells and CD14+ monocytes was necessary for the induction of DC differentiation, we cocultured NK cells and monocytes in the same well but separated by a transwell insert, which allows mixing of soluble molecules but not cells. Under these conditions, neither CD14 down-regulation nor CD86 up-regulation was detected, indicating that cell contact is required for DCNK differentiation (Figure 4A). On the other hand, up-regulation of DC-SIGN and CD40 was still evident, suggesting that soluble factor(s) are responsible for these changes. Thus, the mechanism by which NK cells mediate monocyte differentiation into DCs seems to be dependent partly on cell contact and partly on soluble factors.
Differentiation of DCNK depends on direct contact between NK cells and monocytes and is mediated by GM-CSF and CD40L expressed by NK cells. (A) CD14+ monocytes were cultured in medium alone (filled gray histogram), in direct contact with NK cells (- - -), or with NK cells on opposite sides of a transwell membrane (–). Cells were stained on day 6 using mAb against CD14, CD86, DC-SIGN and CD40. A combined gate was set on monocytes/DCs based on the FSC:SSC profiles and NK cells were excluded from this gate. Results are representative of 3 separate experiments. (B) Isolated CD56bright (•) and CD56dim NK cells (■) derived from PB were cultured at 105 cells/well for 72 hours in medium alone, or in the presence of various concentrations of IL-15 as indicated. GM-CSF in the cell-free culture supernatant was measured by ELISA. The data show mean (± SD) of 3 separate experiments. (C) CD14+ monocytes were cocultured with autologous NK cells in medium alone, or medium containing mouse IgG1 isotype control Ab, or in the presence of neutralizing mAb against either GM-CSF or CD154 as indicated on the top of each contour graph. Cells were stained on day 6 using mAb against DC-SIGN, CD40, CD80, and CD14. A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles, and quadrants are set according to the isotype control staining. Numbers indicate percentage of cells within each quadrant. Results are representative of 2 separate experiments.
Differentiation of DCNK depends on direct contact between NK cells and monocytes and is mediated by GM-CSF and CD40L expressed by NK cells. (A) CD14+ monocytes were cultured in medium alone (filled gray histogram), in direct contact with NK cells (- - -), or with NK cells on opposite sides of a transwell membrane (–). Cells were stained on day 6 using mAb against CD14, CD86, DC-SIGN and CD40. A combined gate was set on monocytes/DCs based on the FSC:SSC profiles and NK cells were excluded from this gate. Results are representative of 3 separate experiments. (B) Isolated CD56bright (•) and CD56dim NK cells (■) derived from PB were cultured at 105 cells/well for 72 hours in medium alone, or in the presence of various concentrations of IL-15 as indicated. GM-CSF in the cell-free culture supernatant was measured by ELISA. The data show mean (± SD) of 3 separate experiments. (C) CD14+ monocytes were cocultured with autologous NK cells in medium alone, or medium containing mouse IgG1 isotype control Ab, or in the presence of neutralizing mAb against either GM-CSF or CD154 as indicated on the top of each contour graph. Cells were stained on day 6 using mAb against DC-SIGN, CD40, CD80, and CD14. A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles, and quadrants are set according to the isotype control staining. Numbers indicate percentage of cells within each quadrant. Results are representative of 2 separate experiments.
Subsequent studies were carried out to determine which soluble and cell-surface factors are required for DCNK differentiation. The first cytokine studied was GM-CSF, which is known to induce DC differentiation.2,38 Unstimulated NK cells secrete minimal amounts of GM-CSF (Figure 4B). However, upon IL-15 stimulation, the CD56bright NK-cell subset secreted higher levels of GM-CSF compared with the CD56dim NK-cell subset (Figure 4B). Blocking endogenous GM-CSF with a neutralizing mAb was sufficient to achieve complete abrogation of NK cell–mediated up-regulation of CD40 and DC-SIGN on monocytes (Figure 4C). Moreover, the monocytes retained their high CD14 expression, indicating that GM-CSF produced during coculture between NK cells and monocytes is required for monocyte differentiation into DC. Blocking IFNγ or TNFα using soluble receptors resulted in only minor changes in CD40 and DC-SIGN expression (data not shown). In the absence of NK cells, none of the blocking mAbs or soluble receptors had any effect on monocytes (data not shown).
CD154 plays a central role in differentiation and activation of DCs.39 Because the receptor for CD154 (ie, CD40) is consistently up-regulated on monocytes cultured in the presence of NK cells, we investigated the possible role of CD154/CD40 interaction in DCNK differentiation. In the presence of a neutralizing antibody against CD154, the CD14 expression level remained high on monocytes cocultured with NK cells (Figure 4C). Reduced levels of both CD40 and DC-SIGN were also seen, whereas the anti-CD154 mAb had no effect on monocytes alone (data not shown). Therefore, interaction between CD154 on NK cells and CD40 on monocytes plays a key role in NK cell–dependent differentiation of monocytes into DC.
NK cells colocalized with monocytes in inflamed synovial tissue and induced their differentiation into DCNK in vitro
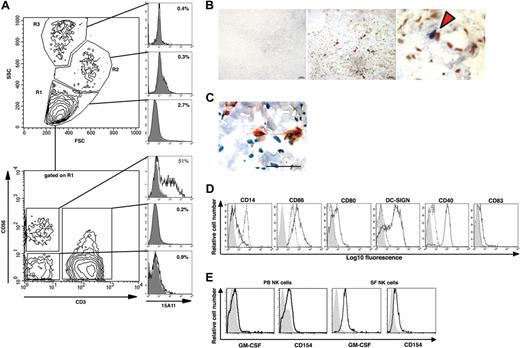
In accordance with previous reports15,40 we found that most freshly isolated NK cells present in SF lack killer immunoglobulin-like receptors (KIR) molecules and express CD56bright (data not shown). CD56 (ie, neural cell adhesion molecule) is not only found on NK cells. It is also expressed by subsets of T cells as well as by some neural tissues. Therefore, to identify NK cells in synovial tissue by immunohistochemistry, we produced a human NK cell–specific mAb (15A11) that binds to human NK cells and shows minimal binding to other cells isolated from either SF or PB (Figure 5A; Figure S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article). Using this mAb, we found NK cells in close cell-to-cell contact with CD14+ monocytes and DC-LAMP+ DC in the RA synovial membrane (Figure 5B,C, respectively). When IL-15 activated SF NK cells were cocultured with autologous monocytes, by day 3 we already had observed a complete down-regulation of CD14, marked up-regulation of CD86, CD80, DC-SIGN, and CD40 (Figure 5D), and the cells acquired typical DC morphology (data not shown). Although CD154 could be detected on both IL-15 stimulated CD56bright PB NK cells and SF NK cells, GM-CSF expression was mainly observed on SF NK cells, as assessed by flow cytometry (Figure 5E).
NK cells colocalize with monocytes and DCs in the RA synovium and induce differentiation of CD14+ monocytes into DCNK in vitro. (A) 15A11 staining pattern on cells isolated from SF of a patient with RA. The 3 gates (ie, R1, R2, and R3) set based on FSC and SSC profiles shown on the top contour plot, and the corresponding top 3 normalized histogram overlay plots show that 15A11 primarily detects cells within a lymphocyte gated (ie R1) population. The bottom contour plot shows CD56 and CD3 expression on cells gated on SF lymphocytes (ie R1), and the corresponding 3 normalized histogram overlay plots show that 15A11 binds to 51% of SF-NK cells (CD3−CD56+), whereas it shows minimal binding to SF-T cells (CD3+), or to CD3−CD56− SF cells. In each histogram, the bold line indicates 15A11 staining, and the filled gray histogram indicates IgM-isotype control staining gated on respective population. An equal number of cells were used for staining with isotype and 15A11 versus CD3 and CD56. (B) The middle panel shows double staining of RA synovium using 15A11 mAb and anti-CD14 mAb. Red arrowheads indicate areas where NK cells (blue) and monocytes (red) are found in close apposition. The bar equals 50 μm. The right panel shows a higher magnification of the area marked with an asterisk in the central panel. The left panel shows staining of an adjacent RA section using isotype control antibodies. (C) Cell contact between NK cells and DCs in inflamed synovial tissue of a representative RA patient. Double staining using 15A11 mAb shows colocalization of 2 NK cells (red) with a DC-LAMP+ DC (brown). The tissue section has been counterstained with hematoxylin (blue) and the bar equals 50 μm. (D) CD14+ monocytes were cultured alone (- - -) or together with IL-15–stimulated SF NK cells (–). Cells were stained on day 3 for surface expression of CD14, CD86, CD80, DC-SIGN, CD40, and CD83 as indicated. DCs are gated based on their FSC:SSC profiles, and NK cells are excluded by gating on CD56+ cells. The filled gray histograms represent the isotype control staining. In each normalized histogram overlay, an equal number of cells were used for staining with isotype versus specific mAb. (E) NK-cell surface expression of GM-CSF and CD154 on PB CD56bright NK cells (left 2 panels) and SF NK cells (right 2 panels). NK cells maintained in IL-15 were stained with a mAb against human GM-CSF or CD154 (bold lines, as indicated) or with an isotype control Ab (filled gray histogram).
NK cells colocalize with monocytes and DCs in the RA synovium and induce differentiation of CD14+ monocytes into DCNK in vitro. (A) 15A11 staining pattern on cells isolated from SF of a patient with RA. The 3 gates (ie, R1, R2, and R3) set based on FSC and SSC profiles shown on the top contour plot, and the corresponding top 3 normalized histogram overlay plots show that 15A11 primarily detects cells within a lymphocyte gated (ie R1) population. The bottom contour plot shows CD56 and CD3 expression on cells gated on SF lymphocytes (ie R1), and the corresponding 3 normalized histogram overlay plots show that 15A11 binds to 51% of SF-NK cells (CD3−CD56+), whereas it shows minimal binding to SF-T cells (CD3+), or to CD3−CD56− SF cells. In each histogram, the bold line indicates 15A11 staining, and the filled gray histogram indicates IgM-isotype control staining gated on respective population. An equal number of cells were used for staining with isotype and 15A11 versus CD3 and CD56. (B) The middle panel shows double staining of RA synovium using 15A11 mAb and anti-CD14 mAb. Red arrowheads indicate areas where NK cells (blue) and monocytes (red) are found in close apposition. The bar equals 50 μm. The right panel shows a higher magnification of the area marked with an asterisk in the central panel. The left panel shows staining of an adjacent RA section using isotype control antibodies. (C) Cell contact between NK cells and DCs in inflamed synovial tissue of a representative RA patient. Double staining using 15A11 mAb shows colocalization of 2 NK cells (red) with a DC-LAMP+ DC (brown). The tissue section has been counterstained with hematoxylin (blue) and the bar equals 50 μm. (D) CD14+ monocytes were cultured alone (- - -) or together with IL-15–stimulated SF NK cells (–). Cells were stained on day 3 for surface expression of CD14, CD86, CD80, DC-SIGN, CD40, and CD83 as indicated. DCs are gated based on their FSC:SSC profiles, and NK cells are excluded by gating on CD56+ cells. The filled gray histograms represent the isotype control staining. In each normalized histogram overlay, an equal number of cells were used for staining with isotype versus specific mAb. (E) NK-cell surface expression of GM-CSF and CD154 on PB CD56bright NK cells (left 2 panels) and SF NK cells (right 2 panels). NK cells maintained in IL-15 were stained with a mAb against human GM-CSF or CD154 (bold lines, as indicated) or with an isotype control Ab (filled gray histogram).
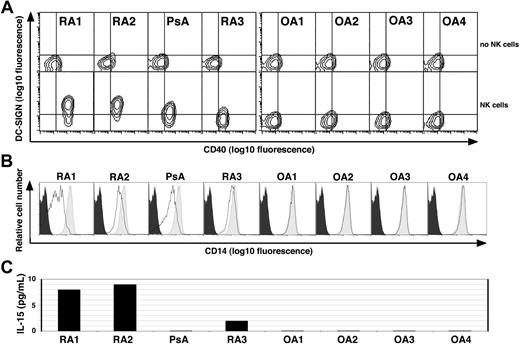
SF from patients with immune-mediated arthritis induced the formation of DCNK from monocytes in the presence of freshly isolated autologous NK cells
Although the inflamed synovium is a rich source of proinflammatory mediators, SF obtained from patients with either RA, PsA, or OA did not by itself promote DC differentiation from monocytes (Figure 6, top row). However, when SF obtained from the inflamed joints of patients with immune-mediated arthritis (ie, RA or PsA), but not from OA, was added to monocytes in the presence of NK cells, they differentiated into DCNK as indicated by increased DC-SIGN and CD40 levels, as well as down-modulated CD14 levels (Figure 6A, bottom row, and 6B, respectively). Moreover, soluble IL-15 was detected in 3 of the 4 SF samples obtained from patients with immune-mediated arthritis but was not detected in the SF of the patients with OA (Figure 6C).
Synovial fluid derived from patients with RA and PsA, but not OA, induces monocyte differentiation into DCs in the presence of NK cells. (A) CD14+ PB monocytes derived from a healthy donor were cultured alone (top row) or together with freshly isolated autologous PB NK cells (bottom row) in medium containing 10% cell-free SF derived from patients with RA (n = 3), PsA (n = 1) or OA (n = 4) as indicated on the top. Cells were stained on day 4 for DC-SIGN and CD40. A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles, and quadrants are set according to the isotype control staining. One of 2 experiments performed with similar results is shown. (B) The light gray histograms depict the CD14 levels on monocytes derived from healthy donors when cultured alone in the presence of medium supplemented with 10% cell-free SF from the patients in panel A, as indicated. The bold lines indicate the CD14 levels on cells cocultured with autologous NK cells in the presence of 10% SF as indicated. The black histogram indicates the isotype control staining of monocytes cultured in medium alone. (C) The IL-15 levels as measured by ELISA in cell-free SF derived from the patients in panel A as indicated.
Synovial fluid derived from patients with RA and PsA, but not OA, induces monocyte differentiation into DCs in the presence of NK cells. (A) CD14+ PB monocytes derived from a healthy donor were cultured alone (top row) or together with freshly isolated autologous PB NK cells (bottom row) in medium containing 10% cell-free SF derived from patients with RA (n = 3), PsA (n = 1) or OA (n = 4) as indicated on the top. Cells were stained on day 4 for DC-SIGN and CD40. A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles, and quadrants are set according to the isotype control staining. One of 2 experiments performed with similar results is shown. (B) The light gray histograms depict the CD14 levels on monocytes derived from healthy donors when cultured alone in the presence of medium supplemented with 10% cell-free SF from the patients in panel A, as indicated. The bold lines indicate the CD14 levels on cells cocultured with autologous NK cells in the presence of 10% SF as indicated. The black histogram indicates the isotype control staining of monocytes cultured in medium alone. (C) The IL-15 levels as measured by ELISA in cell-free SF derived from the patients in panel A as indicated.
Discussion
This study demonstrates that activated KIR−CD56bright NK cells obtained from either normal PB or from SF of patients with inflammatory arthritis are capable of inducing the differentiation of CD14+ monocytes into DCs (here termed DCNK). In the presence of such NK cells, monocytes gradually lost expression of the monocyte-specific CD14 marker and acquired several DC-associated differentiation markers. DCNK also expressed high levels of DC-LAMP (CD208), a molecule typically associated with activated or mature DC in lymph nodes and synovial tissue.35,41 It is noteworthy that cell-free SF from patients with inflammatory arthritis could drive DC differentiation from monocytes provided that NK cells were present. These phenotypic changes in monocytes coincided with an almost complete loss of their capacity to phagocytose large particles, whereas their capacity to endocytose small antigens was enhanced. The DCNK were efficient in stimulating T-cell responses, as shown by their capacity to trigger naive CD4+ T-cell proliferation and IFNγ synthesis.
Several recent studies have analyzed the capacity of NK cells to interact with DCs, but none explored the ability of NK cells to induce DC differentiation from monocytes.27,–29,42 In these studies, immature DCs were generated in vitro from monocytes that had initially been enriched by either plastic adherence or selection using anti-CD14–coated beads and were subsequently cultured in GM-CSF and IL-4 for several days before NK-cell coculture. Here we have cocultured activated NK cells and monocytes ex vivo from day 0, and we have analyzed the potential of CD14+ monocytes, which are not susceptible to NK cell–mediated cytotoxicity (data not shown), to differentiate into DC in the absence of exogenous GM-CSF and IL-4.
To our knowledge, this is the first report to describe NK cell–dependent generation of DCs from monocytes. This process most likely occurs in lymph nodes and inflamed tissues, where both monocytes16,18,43,,–46 and CD56bright NK cells12,14,15,19,47 are known to accumulate. Moreover, such NK cells produce GM-CSF, here shown to be the critical cytokine mediating the development of DCs. However, to initiate DCs differentiation from monocytes, the CD56bright NK cells must be activated, and our data indicate that either exogenous IL-15, or synovial fluid, is sufficient to induce this process. It is noteworthy that Cooper et al37 showed that not only is IL-15 stimulation sufficient to induce GM-CSF in CD56bright NK cells but it is also required for murine NK-cell survival in vivo48 and induction of human lymph node NK-cell activation by splenic DCs ex vivo.17 Although IL-15 is not produced by NK cells, it is produced by many cell types, including monocytes and DCs,17,49 which also express IL-15 receptor α (IL15Rα),50 necessary for transpresentation of IL-15 to neighboring NK cells.17,51,–53 Moreover, GM-CSF is present at significant levels in the RA synovial fluid,54 and GM-CSF stimulation of monocytes results in their rapid cell-surface expression of biologically active IL-15,55 which can thus be presented to neighboring NK cells. Based on this information and our results, we hypothesize that monocyte-derived IL-15 and NK cell–derived GM-CSF copresented on cellular contact might constitute a positive feedback loop in the NK-cell activation and monocyte differentiation pathways. Such a process could explain how NK cells can modulate monocyte differentiation not only during host responses to foreign pathogens but also during healthy homeostasis in lymph nodes and in certain clinical settings, such as in RA.
The finding that NK cells drive DC differentiation from monocytes may seem to be at odds with earlier reports showing that NK cells can deplete immature DCs either by killing them or by inducing their maturation through cytokine release.27,–29 These studies analyzed immature DCs propagated from monocytes in GM-CSF and IL-4 before NK cell coculture in vitro, and it has been suggested that this activity of NK cells may help to shape a more mature DC repertoire in vivo (reviewed by Moretta56 ). In contrast, we show here that NK cells induce the generation of immature DCs, which seem at least as differentiated as DCGM/IL4 as judged by their morphology, phenotype, endocytic and phagocytic capacity, and their ability to stimulate T-cell proliferation and T-cell cytokine release. We have not determined whether DCNK become susceptible to cell-mediated cytotoxicity upon coculture with freshly isolated NK cells. This seems unlikely, because DCNK remain in the wells even after prolonged (up to 2 weeks) coculture with IL-15 stimulated NK cells. Nonetheless, the effects of NK cells on DCs may vary with their respective activation states and culture conditions.28,29 We used low numbers of NK cells, and we cannot exclude the possibility that at some point during the differentiation process from monocyte to DCNK, some cells pass through a stage at which they may be susceptible to NK cell–mediated lysis.
Differences in experimental procedures may explain why a recent study showed that 25% to 60% of monocytes stimulated with IL-15 not only expressed DC-SIGN but also differentiated into macrophages,57 whereas in our studies, IL-15 alone had no effect on monocyte morphology, phagocytic capacity, or induction of DC-SIGN. We used highly purified CD14+ monocytes as the starting population, whereas Krutzik et al57 obtained monocytes by plastic adherence known to result in a heterogenous cell population.58
In conclusion, our findings reveal a previously unknown biological function of NK cells: the induction of DC differentiation from monocytes. This process likely occurs at sites of chronic inflammation such as the RA joint. Clearly, any in vitro protocol to generate DCs from monocytes cannot completely recapitulate the cytokine and/or cellular environment in which monocytes differentiate into DCs in vivo. Despite this limitation, we propose that DCNK are closely related to DCs present in the RA joint in vivo. This view is supported by (1) the frequent occurrence in inflamed RA tissue of NK cells, CD14+ monocytes and DC-LAMP+ DCs in close contact with one another, and (2) the observation that cell-free SF, or IL-15, initiates formation of TH1-promoting DCs from CD14+ monocytes in vitro, but only in the presence of NK cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs William Robinson and Mark Genovese for help with clinical samples, Donna Jones for secretarial assistance, Claudia Benike for critical review of the manuscript, and Lorna Torentino for assistance with cell sorting.
This work was supported by National Institutes of Health grants HL57443, AR051748, and AI055468. C.T.M. was supported by a PhD fellowship from Fundação para a Ciência e a Tecnologia (PRAXIS XXI/BD/4047/94). U.P. and I.H.T. were funded in part by the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG] grant no. Ta297).
National Institutes of Health
Authorship
Contribution: K.S., A.L.Z., and P.C. designed the study. A.L.Z., P.C., C.T.M., U.P., I.H.T., and K.S. performed research and analyzed data. A.L.Z., P.C., K.S., and E.G.E. wrote the manuscript. I.H.T., W.H., and L.K. organized collection of patient samples. All authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kalle Söderström, Department of Pathology, Stanford University, 3373 Hillview Avenue, Palo Alto, CA 94304-1204; e-mail:kalsod@stanford.edu.

![Figure 3. DCNK take up antigen by endocytosis, lose phagocytic capacity, and exhibit potent T-cell stimulatory ability. (A) Antigen uptake by endocytosis as assessed by adding DQ-OVA to monocytes cultured in medium alone (filled gray histogram) or in the presence of GM-CSF and IL-4 (- - -), or monocytes cocultured with an NK-cell clone (–). Cells were incubated with DQ-OVA overnight and subsequently analyzed by flow cytometry. A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles. The histogram of NK cells alone (using a combined gate of CD56+ and FSC:SSC) is shown to indicate the fluorescence intensity of nonendocytic NK cells (· · ·). (B) Phagocytic activity, as measured by uptake of FITC-conjugated latex beads of monocytes cultured in medium alone, with IL-15, with NK cells in medium alone (monocytes NKs cells), NK cells and IL-15 (DCNK), or GM-CSF and IL-4 (DCGM/IL4). A combined gate was set on monocytes/DCs based on their CD56− and FSC:SSC profiles. Percentages indicate phagocyte activity. Results are representative of 2 separate experiments. (C) DCNK induce potent proliferation of naive allogeneic CD4+ T cells. DCGM/IL4 (circles) and DCNK (triangles) were isolated on day 6 and stimulated with TNFα (gray, 10 ng/mL), LPS (black, 1 μg/mL), or left in medium alone overnight (white). The DC were thereafter irradiated (3000 rad) and incubated at graded doses with naive CD4+ T cells as indicated. After 7 days, the cells were pulsed with [3H]thymidine and proliferation is expressed in counts per minute (cpm) × 103 (mean ± SD of triplicate cultures). (D) DCNK or DCGM/IL4 were stimulated overnight with either 10 ng/mL TNFα or medium alone before coculture with T cells (1:1 ratio) as indicated. Assessment of intracellular IFNγ (top) and IL-4 (bottom) was done using PE-conjugated anti-IFNγ and anti–IL-4 mAbs after a 72-hour culture, and the percentages of cytokine positive cells are indicated in each dot plot. IFNγ or IL-4 produced by T cells without DC stimulation, or by monocyte stimulation, was less than 0.1%. The vertical line is set to indicate the background of the isotype control staining. Results are representative of 3 separate experiments. (E) Phenotypic maturation of DCNK (top panels) and DCGM/IL4 (bottom panels). Parallel cultures of DCNK and DCGM/IL4 were harvested on day 6 and stimulated with 10 ng/mL TNFα, or kept in medium alone, for an additional 48 hours. Cell surface expression of CD40, CD80, CD83, and CD86 on TNFα-matured DC (–) is compared with immature DC (- - -). Gray histograms show staining with isotype control antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-076364/2/m_zh80210708520003.jpeg?Expires=1769108785&Signature=HxUhTYSF7t4LX4uLkldgINZTm3VGGr7L6~rtwjt~N4kkUDMXS2~wj2il8vqhW~Fu-4N38J4z6J7kkQxSTuNbvkId0Y0Jb36p~eGMwigXg40dO0BN0TBGFpKO5Nq9k~JJBc~oJjCdKeV8MKxGbNFSbftpZZm9uwUaIy2OTAEMhzScmT8hS0sGD4sJo6pQug~Cnj9rv8wkvsTMnY9c4DTtWTFX0wD42qttMOzzw9ahf4ABCMYXDhLyNXGQfQZCxqFr4PnJZD9IdIlmNJYQForCMfN11IJ4hYkOgwNCD--uLCMEHn7ZtjA46YZgZI0bDg47-wuKFbatLHIQc1wdZeQjmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)