Collagen activates platelets through an intracellular signaling cascade downstream of glycoprotein VI (GPVI). We have investigated the contribution of hematopoietic lineage cell–specific protein 1 (HS1) downstream of GPVI in platelet activation. Stimulation of GPVI leads to tyrosine phosphorylation of HS1, which is blocked by Src-family kinase inhibitors. Coimmunoprecipitation experiments revealed that HS1 associates with Syk and phosphatidylinositol 3-kinases. HS1-null mice displayed increased bleeding times and increased time to occlusion in the FeCl3 in vivo thrombosis model compared with their wild-type littermates. In addition, aggregation and secretion responses were diminished in HS1-null mouse platelets after stimulation of GPVI and protease-activated receptor 4 (PAR-4) agonists compared with wild-type littermate mouse platelets. Finally, Akt phosphorylation was diminished after GPVI or PAR-4 stimulation in platelets from HS1-null mice compared with their wild-type littermates. These results demonstrate that phosphorylation of the HS1 protein occurs downstream of GPVI stimulation and that HS1 plays a significant functional role in platelet activation downstream of GPVI and PARs.
Introduction
Platelet activation is pivotal in the arrest of bleeding after vessel injury. Several pathways originating from G-protein–coupled receptors and integrins located on the platelet membrane contribute to platelet activation. For example, injury to the endothelium of a blood vessel leads to the release of tissue factor and exposure of subendothelial collagen, which initiates platelet activation. Collagen binds to platelets directly via the α2β1 integrin and the glycoprotein VI (GPVI) receptor or indirectly through von Willebrand factor and GPIb/V/IX complex.1,,,–5
The binding of collagen to the GPVI receptor results in an intracellular signaling cascade that leads to platelet activation.6,7 The GPVI receptor, a member of the immunoglobulin (Ig) superfamily, is coexpressed with Fc receptor γ (FcRγ) chain on platelets and serves as a single functional unit.8 After the binding of collagen to GPVI, the FcRγ chain is phosphorylated by Src kinases on the tyrosine residues of its immunoreceptor tyrosine-based activation motif (ITAM),9,10 which, in turn, promotes the association of Syk kinase; consequently, Syk undergoes autophosphorylation. Activated Syk subsequently phosphorylates and activates phospholipase Cγ2 (PLCγ2) through a cascade of signaling molecules involving LAT (linker for T-cell activation), phosphatidylinositol 3-kinase (PI3K), and Bruton tyrosine kinase. Activation of PLCγ2 leads to the rise in intracellular calcium and protein kinase C (PKC) activation, which results in secretion of dense granules, generation of thromboxane, and activation of the αIIbβ3 integrin, which leads to aggregation.11
Activation of B and T lymphocytes leads to phosphorylation of a protein exclusively expressed in cells of hematopoietic lineage that is termed hematopoietic lineage cell–specific protein 1 (HS1).12 HS1 is a 486-amino acid–long 75-kDa hydrophilic protein.12 It is predominantly located in cytoplasm but after activation through tyrosine phosphorylation at residues 397, 378, and 22213,14 translocates to plasma membrane. The protein also contains a HAX1 (HS-associated protein X-1)–binding site, an Src homology 3 (SH3) domain, a proline-rich region, and 3 additional phosphorylation sites.14,15 These sites are sequentially phosphorylated after B- and T-cell receptor cross-linking. After the phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM), the nonreceptor tyrosine kinase Syk is phosphorylated and activated. Phosphorylated Syk binds to HS1, which results in tyrosine phosphorylation of HS1 at residues 397 and 378. Syk then dissociates from HS1, which allows Src-family kinases to bind to HS1 via its SH2 or SH3 domain. Src subsequently phosphorylates HS1 at tyrosine residue 222, which results in activation of the HS1 protein. Functionally, the HS1 protein has been shown to be involved in proliferation and apoptosis downstream of B- and T-cell receptor activation.14,16,–18
Although HS1 has been reported to be expressed and phosphorylated in platelets after thrombin stimulation,19 little information is known about the function of this protein in platelet activation. Because of its tissue-specific expression and putative cell-type functions, we hypothesized that HS1 regulates platelet activation downstream of the Ig-like receptor, viz, GPVI. In this study we demonstrate that HS1 phosphorylation depends on Src-family kinases after activation of the GPVI receptor. More significantly, our studies, in which we used a genetic mouse model, revealed that HS1 plays an important role in GPVI-induced platelet activation and in vivo thrombus formation.
Materials and methods
Materials
Apyrase (type VII), bovine serum albumin (fraction V), acetylsalicylic acid, and REDExtract-N-Ampt tissue polymerase chain reaction (PCR) kit were obtained from Sigma (St Louis, MO). Convulxin was purchased from CenterChem (Norwalk, CT). The HS1 antibody was obtained from BD Biosciences (San Jose, CA), and the p110 PI3K, normal mouse IgG, normal rabbit IgG, and protein G-plus Sepharose beads were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). The JON/A-PE–labeled antibody was purchased from Emfret Technologies (Eibelstadt, Germany). The Chronolume reagent was purchased from Chrono-Log (Havertown, PA). Monoclonal phosphotyrosine antibody (clone 4G10) was purchased from Upstate Biotechnologies (Lake Placid, NY). Cell lysis buffer (20 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.5/150 mM NaCl/1 mM Na2EDTA [ethylenediaminetetraacetic acid]/1 mM EGTA [ethyleneglycoltetraacetic acid]/1% triton/2.5 mM sodium pyrophosphate/1 mM β-glycerophosphate/1 mM Na3VO4/1 μg/mL leupeptin), Akt, PI3K, and Syk antibodies were obtained from Cell Signaling Technologies (Beverly, MA). Oligonucleotides to the HS1 gene were purchased from Integrated DNA Technologies (Coralville, IA). AYPGKF was acquired as a custom-made peptide from New England Peptide (Fitchburg, MA). The HS1-deficient mice18 were provided by D.K. and backcrossed to C57BL/6 mice for 10 generations (at the Research Institute for Biological Sciences, Tokyo University of Science) before they were intercrossed to generate homozygotes. The P2Y1-null mice were generated in our laboratory.20
Isolation of human platelets
Informed consent was obtained in accordance with the Declaration of Helsinki, and whole blood was drawn from healthy human volunteers into tubes containing a one-sixth volume of ACD (2.5 g sodium citrate, 1.5 g citric acid, and 2 g glucose in 100 mL deionized water) reagent. Blood was centrifuged (Eppendorf [Hamburg, Germany] 5810R centrifuge) at 230g for 20 minutes at room temperature to obtain platelet-rich plasma (PRP). PRP was incubated with 1 mM acetylsalicylic acid for 30 minutes at 37°C. The PRP then was centrifuged for 10 minutes at 980g at room temperature to pellet the platelets. Platelets were resuspended in Tyrode buffer (138 mM NaCl/2.7 mM KCl/1 mM MgCl2/3 mM NaH2PO4/5 mM glucose/10 mM Hepes [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4/0.2% bovine serum albumin) containing 0.01 U/mL apyrase. Cells were counted using a Coulter Z1 particle counter (Beckman Coulter, Fullerton, CA), and the concentration of cells was adjusted to 4 × 108 platelets per mL. All experiments with washed platelets were performed in the absence of extracellular calcium unless otherwise mentioned. The protocols for use of human participants and animals were approved by the Institutional Review Board of Temple University Medical School.
Isolation of mouse platelets
Blood was collected from the vena cava of anesthetized mice into syringes containing a one-tenth blood volume of 3.8% sodium citrate as anticoagulant. Red blood cells were removed by centrifugation at 100g for 10 minutes. PRP was recovered, and platelets were pelleted at 400g for 10 minutes. The platelet pellet was resuspended in Tyrode buffer (pH 7.4) containing 0.01 U/mL apyrase. The washed platelets were subsequently used for experiments.
Aggregometry
After the platelets were prepared as described in “Materials and methods, Isolation of human platelets” and “Isolation of mouse platelets,” aggregation of 500 μL washed human platelets or 250 μL washed mouse platelets were analyzed using a PICA lumiaggregometer (Chrono-Log). Aggregation was measured using light transmission under mixing conditions (900 rpm) at 37°C. Agonists were added for platelet stimulation. Each sample was allowed to aggregate for at least 3 minutes. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/s.
Immunoprecipitation and Western blot analysis
Platelets were stimulated with agonists in the presence or absence of inhibitors for the appropriate time, and the reaction was stopped by the addition of equal volumes of the 2× cell lysis buffer. The lysates were incubated for 30 minutes in ice for completion of the lysis. The cell lysates were isolated, immunoprecipitating antibody was added at 1:100 dilution and incubated for 2 hours at 4°C, and lysates were processed for sodium dodecyl sulfate gel electrophoresis and immunoblotting as described previously.21 Alternatively, the samples were directly used for immunoblotting using the primary antibodies followed by alkaline phosphatase–coupled second antibodies per established protocols. Finally, the membranes were incubated with CDP-Star chemiluminescent substrate (Tropix, Bedford, MA) for 10 minutes at room temperature, and immunoreactivity was detected using a Fuji Film (Tokyo, Japan) LAS-1000 CH luminescent image analyzer.
Measurement of platelet secretion
Platelet secretion was determined by measuring the release of adenosine triphosphate (ATP) using the Chronolume reagent. The activation of platelets was performed in a lumiaggregometer at 37°C with stirring at 900 rpm, and the secretion was measured and expressed as nanomoles of ATP released per 108 platelets. In experiments in which inhibitors were used, the platelet samples were preincubated with the inhibitors for 10 minutes at 37°C before the addition of agonists.
Genotyping
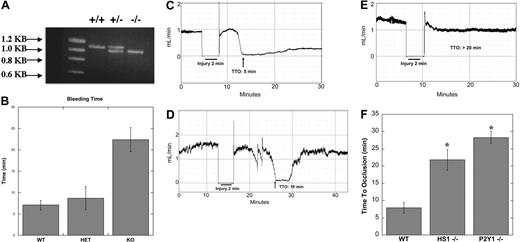
Tails from the mice were cut 0.3 cm from the tip. DNA from the tail was extracted and amplified using the REDExtract-N-AMP tissue PCR kit with primers pHS1–3′KO-S, HS1-KO-end03′, and LAC-Z-3. After PCR, the DNA was run on a 0.8% agarose gel for 30 minutes. The DNA was detected using a Fuji Film LAS-1000 CH luminescent image analyzer.
Measurement of mouse bleeding times
Wild-type, heterozygous, and knockout mice were bled at 6 to 8 weeks of age by cutting 0.3 cm from the end of the tail and then submerging it in 0.9% saline. Once the tail had been cut, a timer was started and continued to run until stoppage of bleeding. When no blood was seen dripping from the end of the tail, it was deemed that the bleeding had stopped and the time was recorded. Bleeding was stopped in all cases at 30 minutes.
In vivo thrombosis model
Adult mice (6-8 weeks old: weight, ∼25 g) were anesthetized by intraperitoneal injection of pentobarbital (40 mg/kg). Experimental groups consisted of HS1+/+, HS1−/−, and P2Y1−/− mice. The left carotid artery was exposed surgically, and a miniature Doppler flow probe (model 0.7PSB; Transonic Systems, Ithaca, NY) was placed on the surface of the artery. Normal saline was placed in the surgical wound to allow Doppler monitoring, and baseline blood flow was recorded using a Transonic T402 flow meter. Thereafter, Whatmann (Maidstone, United Kingdom) filter paper (1 × 1 mm) saturated with 10% FeCl3 was applied to the adventitial surface of the carotid artery, immediately proximal to the flow probe. After 2 minutes, the filter paper was removed, saline solution was again placed in the wound, and carotid blood flow was monitored (ie, it was not possible to monitor carotid artery blood flow during the application of FeCl3). Time to thrombotic occlusion after initiation of arterial injury was defined as the time required for blood flow to decline to 0 mL/min. If the carotid artery was observed to be thrombosed at the earliest time point that flow could be monitored after initiation of the injury (ie, 2 minutes), time to occlusion was recorded as less than 2 minutes. The operator was blinded to mouse genotype while performing all experiments.
Flow cytometry
All determinations were performed with a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Washed platelets were analyzed before and after activation with convulxin, and surface expression of JON/A was examined. Platelets were stimulated with the appropriate agonist at 37°C in nonstirring conditions. After 3 minutes of stimulation, the activated washed mouse platelets were added to Tyrode buffer and the αIIbβ3 antibody JON/A-PE in the presence of 1 mM CaCl2. The platelets were incubated with the antibody for 15 minutes, and the reaction was stopped by adding 400 μL phosphate-buffered saline. The samples were analyzed within 15 minutes of completing the reaction by flow cytometry. Light-scatter and fluorescence data from 10 000 platelet events were collected with all detectors in logarithmic mode.
Results
HS1 undergoes tyrosine phosphorylation after GPVI activation
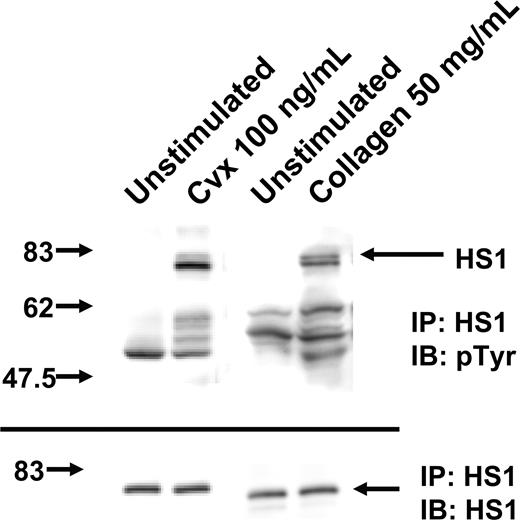
HS1 is known to be phosphorylated downstream of antigen receptors in lymphocytes.13,14 To investigate the role of HS1 in platelet activation after GPVI activation, we first examined the activation of HS1 by measuring its phosphorylation status. The GPVI-selective agonist convulxin (a snake venom protein that belongs to the heterodimeric C-type lectin family)22 binds directly to GPVI, which leads to the phosphorylation of tyrosine residues on the ITAM and activation of PLCγ2 and several other proteins.22,23 Lysates from washed platelets, immunoprecipitated for HS1 and immunoblotted for p-Y using the 4G10 antibody, showed no HS1 phosphorylation under unstimulated conditions. However, after treatment with 100 ng/mL convulxin or 50 μg/mL collagen, HS1 phosphorylation occurred (Figure 1). Furthermore, several other tyrosine-phosphorylated proteins were also coimmunoprecipitated along with activated HS1 by stimulation with either collagen or convulxin. Thus, HS1 undergoes tyrosine phosphorylation after activation of the GPVI receptor. The specific association of other tyrosine-phosphorylated proteins with activated HS1 is consistent with its putative functions as a signaling adapter molecule. Tyrosine phosphorylation of HS1 was also observed in washed mouse platelets (data not shown).
Effect of GPVI activation on tyrosine phosphorylation of HS1. Washed and aspirin-treated platelets were stimulated with convulxin (Cvx) or collagen for 30 seconds at 37°C as indicated. HS1 was immunoprecipitated (IP) as described, and the samples were analyzed for tyrosine phosphorylation (pTyr) by Western blotting (IB) using the monoclonal phosphotyrosine (4G10) antibody. Equal lane loading was assured by probing the samples with the HS1 antibody. The Western blot shown is representative of experiments performed using platelets from 3 different donors.
Effect of GPVI activation on tyrosine phosphorylation of HS1. Washed and aspirin-treated platelets were stimulated with convulxin (Cvx) or collagen for 30 seconds at 37°C as indicated. HS1 was immunoprecipitated (IP) as described, and the samples were analyzed for tyrosine phosphorylation (pTyr) by Western blotting (IB) using the monoclonal phosphotyrosine (4G10) antibody. Equal lane loading was assured by probing the samples with the HS1 antibody. The Western blot shown is representative of experiments performed using platelets from 3 different donors.
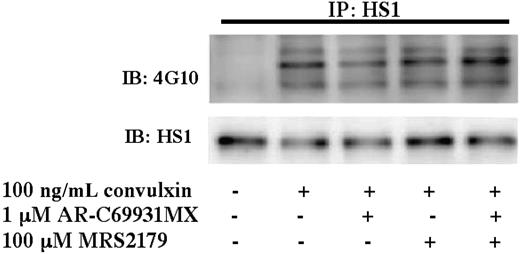
Because GPVI activation leads to the secretion of adenosine 5′-diphosphate (ADP) and the generation of thromboxane,24 we sought to eliminate their contribution in HS1 activation using selective ADP-receptor antagonists and aspirin-treated platelets, respectively. Platelets were stimulated with 100 ng/mL convulxin in the presence of the P2Y1-receptor antagonist MRS2179, the P2Y12-receptor antagonist AR-C69931M, or both (Figure 2). Antagonizing the P2Y receptors did not affect HS1 phosphorylation after stimulation with convulxin. Collagen (50 μg/mL)–induced HS1 phosphorylation was also not affected by addition of these antagonists (data not shown). These results indicate that secreted ADP does not affect GPVI-mediated HS1 phosphorylation.
Role of ADP-receptor antagonists in the tyrosine phosphorylation of HS1. Aspirin-treated and washed human platelets were stimulated with 100 ng/mL convulxin in the presence or absence of MRS2179 (10 μM), AR-C69931MX (100 nM), or both at 37°C, and the reaction was stopped by adding 2× cell lysis buffer. The stimulation time for convulxin was 30 seconds. HS1 was immunoprecipitated (IP) as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting (IB) using the monoclonal phosphotyrosine (4G10) antibody. The Western blots shown are representative of experiments performed using platelets from 3 different donors.
Role of ADP-receptor antagonists in the tyrosine phosphorylation of HS1. Aspirin-treated and washed human platelets were stimulated with 100 ng/mL convulxin in the presence or absence of MRS2179 (10 μM), AR-C69931MX (100 nM), or both at 37°C, and the reaction was stopped by adding 2× cell lysis buffer. The stimulation time for convulxin was 30 seconds. HS1 was immunoprecipitated (IP) as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting (IB) using the monoclonal phosphotyrosine (4G10) antibody. The Western blots shown are representative of experiments performed using platelets from 3 different donors.
HS1 associates with Syk and PI3Ks
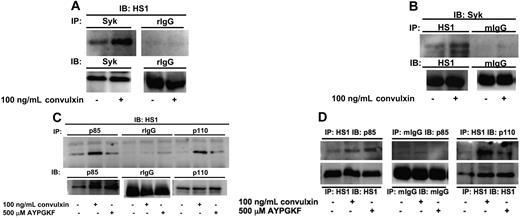
Previous studies have shown that Syk and Src kinases associate and sequentially phosphorylate HS1 at tyrosine residues 378, 397, and 222, respectively.13,14 We investigated the association of HS1 with Syk kinase downstream of GPVI activation. Aspirin-treated platelets were stimulated with 100 ng/mL convulxin, immunoprecipitated for Syk (Figure 3A), and immunoblotted for HS1. We also carried out reverse immunoprecipitations using HS1 antibodies for immunoprecipitation and Syk antibodies for immunoblotting (Figure 3B). Normal IgG (rabbit or mouse) served as a negative control in both experiments. In both cases HS1 associated with Syk after GPVI activation after a 30-second stimulation with convulxin (Figure 3A,B), which indicated that HS1 associates with the nonreceptor tyrosine kinase Syk after platelet activation.
HS1 associates with Syk and PI3Ks after receptor stimulation. Aspirin-treated and washed human platelets were stimulated with agonist at 37°C for 30 seconds. (A-B) Syk kinase (A) or HS1 (B) was immunoprecipitated (IP) as described in “Materials and methods, Immunoprecipitation and Western blot analysis,” and the samples were analyzed for HS1 (A) or Syk (B) by immunoblotting (IB). Rabbit IgG (rIgG) or mouse IgG (mIgG) was used as a control. (C,D) PI3K subunits p85 and p110 (C) or HS1 (D) were immunoprecipitated as described, and the samples were analyzed for HS1 (C) or p85 and p110 (D) by immunoblotting. A negative control with immunoprecipitated normal IgG (mouse or rabbit) was analyzed for comparison. The Western blots shown are representative of experiments performed using platelets from 3 different donors.
HS1 associates with Syk and PI3Ks after receptor stimulation. Aspirin-treated and washed human platelets were stimulated with agonist at 37°C for 30 seconds. (A-B) Syk kinase (A) or HS1 (B) was immunoprecipitated (IP) as described in “Materials and methods, Immunoprecipitation and Western blot analysis,” and the samples were analyzed for HS1 (A) or Syk (B) by immunoblotting (IB). Rabbit IgG (rIgG) or mouse IgG (mIgG) was used as a control. (C,D) PI3K subunits p85 and p110 (C) or HS1 (D) were immunoprecipitated as described, and the samples were analyzed for HS1 (C) or p85 and p110 (D) by immunoblotting. A negative control with immunoprecipitated normal IgG (mouse or rabbit) was analyzed for comparison. The Western blots shown are representative of experiments performed using platelets from 3 different donors.
HS1 has also been shown to associate with other important signal transduction molecules, including PI3K, after cellular activation.25 Hence, we investigated the association of HS1 with PI3K downstream of GPVI and PAR-4 activation. Aspirin-treated platelets were stimulated with 100 ng/mL convulxin or 500 μM AYPGKF, immunoprecipitated for p85 and p110 subunits of PI3K, and immunoblotted for HS1 (Figure 3C). As shown in Figure 3C, HS1 associates with both p85 and p110 subunits of PI3K. However, when normal IgG was used for immunoprecipitations, no association was detected, which ruled out nonspecific immunoprecipitation of HS1. We also confirmed this association between HS1 and PI3K using reverse immunoprecipitation. In this case, we used HS1 antibodies for immunoprecipitation and either p85 or p110 subunit antibodies for immunoblotting. As shown in Figure 3D, HS1 associates with the p85 and p110 subunits of PI3K after platelet stimulation. The association between HS1 and p85 or p110 seems to be stronger after convulxin stimulation compared with AYPGKF stimulation of platelets. These results indicate that HS1-associated PI3K may have a role in platelet activation.
HS1 is phosphorylated by nonreceptor tyrosine kinases
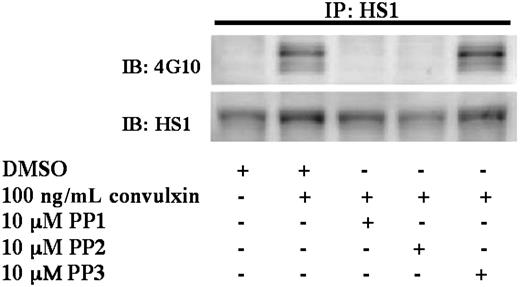
We next investigated the contribution of Src-family kinases in GPVI-mediated HS1 phosphorylation. Aspirin-treated platelets were stimulated with 100 ng/mL convulxin and treated with the Src-family kinase inhibitors 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine 1 (PP1) or 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) and the control compound PP3, which is a structurally similar inactive analog to PP1 and PP2. Inhibition of Src-family kinases by PP1 or PP2 prevents ITAM phosphorylation, which renders Syk inactive. Therefore, treatment with Src inhibitors is predicted to block signaling downstream of GPVI and Syk activation. As shown in Figure 4, the sample treated with PP3 showed similar levels of phosphorylation compared with the positive control. Phosphorylation of HS1, however, was abolished in the samples treated with the Src kinase inhibitors (Figure 4). These results suggest that Src-family kinases are required for HS1 phosphorylation downstream of GPVI activation.
Role of Src-family tyrosine kinases in the tyrosine phosphorylation of HS1. Aspirin-treated and washed human platelets were stimulated with 100 ng/mL convulxin in the presence or absence of PP1 (10 μM), PP2 (10 μM), or the control compound PP3 (10 μM) at 37°C, and the reaction was stopped by adding 2× cell lysis buffer. Dimethyl sulfoxide (DMSO) was used as a vehicle control. The stimulation time for convulxin was 30 seconds, and the incubation time for PP1, PP2, and PP3 was 10 minutes at 37°C. HS1 was immunoprecipitated (IP) as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting (IB) using the monoclonal phosphotyrosine (4G10) antibody. The Western blots shown are representative of experiments performed using platelets from 3 different donors.
Role of Src-family tyrosine kinases in the tyrosine phosphorylation of HS1. Aspirin-treated and washed human platelets were stimulated with 100 ng/mL convulxin in the presence or absence of PP1 (10 μM), PP2 (10 μM), or the control compound PP3 (10 μM) at 37°C, and the reaction was stopped by adding 2× cell lysis buffer. Dimethyl sulfoxide (DMSO) was used as a vehicle control. The stimulation time for convulxin was 30 seconds, and the incubation time for PP1, PP2, and PP3 was 10 minutes at 37°C. HS1 was immunoprecipitated (IP) as described, and the samples were analyzed for tyrosine phosphorylation by Western blotting (IB) using the monoclonal phosphotyrosine (4G10) antibody. The Western blots shown are representative of experiments performed using platelets from 3 different donors.
HS1 deficiency profoundly impairs in vivo platelet function
HS1-null mice were generated, and the function of HS1 was studied in lymphocytes using these mice.18 To evaluate the physiologic consequences of HS1 deficiency on platelet function in vivo, we investigated the effect of this deficiency on bleeding times. In these experiments, 0.3 cm of mouse tails were clipped and submerged in 0.9% saline. DNA from the clipped portions was extracted and genotyped by PCR analysis (Figure 5A). Once the tails were clipped and submerged in saline, time until bleeding stoppage was measured. Wild-type mice stopped bleeding within 5 minutes. HS1 heterozygote mice also halted bleeding at approximately 5 minutes. Conversely, the HS1-null mice bled for at least 20 minutes (Figure 5B). Thus, the knockout mice bled significantly longer than both HS1 heterozygote and wild-type mice, which demonstrated that HS1 deficiency results in a bleeding diathesis. This important finding underscores the potential role of HS1 in the platelet-plug formation under physiologic conditions.
In vivo functional consequences of HS1 deficiency in mice. (A) The mice's tails were clipped 0.3 cm from the end and submerged in 0.9% saline. The clipped portions were isolated and subjected to genotyping of the HS1 locus by PCR. (B) Time until the stoppage of bleeding was measured; the times were then compared with that of the PCR analysis (n > 15). Statistical analysis by ANOVA revealed a P value less than .01 between HS1-deficient and wild-type (WT) mice and HS1-deficient and HS1-heterozygote (HET) mice. Error bars indicate standard error. (C-E) Wild-type (n = 8) (C) and HS1- (n = 11) (D) and P2Y1- (n = 8) (E) deficient mice were injured by 10% FeCl3 for 2 minutes, and time to occlusion (TTO) was measured by optical Doppler flow. (F) Time to 90% occlusion was calculated and graphed. Statistical analysis by t test revealed a P value less than .01 between wild-type and HS1-deficient mice and wild-type and P2Y1-deficient mice. *Statistically significant (P < .01).
In vivo functional consequences of HS1 deficiency in mice. (A) The mice's tails were clipped 0.3 cm from the end and submerged in 0.9% saline. The clipped portions were isolated and subjected to genotyping of the HS1 locus by PCR. (B) Time until the stoppage of bleeding was measured; the times were then compared with that of the PCR analysis (n > 15). Statistical analysis by ANOVA revealed a P value less than .01 between HS1-deficient and wild-type (WT) mice and HS1-deficient and HS1-heterozygote (HET) mice. Error bars indicate standard error. (C-E) Wild-type (n = 8) (C) and HS1- (n = 11) (D) and P2Y1- (n = 8) (E) deficient mice were injured by 10% FeCl3 for 2 minutes, and time to occlusion (TTO) was measured by optical Doppler flow. (F) Time to 90% occlusion was calculated and graphed. Statistical analysis by t test revealed a P value less than .01 between wild-type and HS1-deficient mice and wild-type and P2Y1-deficient mice. *Statistically significant (P < .01).
The identification of a bleeding phenotype in the HS1-null mice led us to investigate whether HS1 participates in the thrombus formation in vivo. Using the FeCl3 carotid artery–injury model of in vivo thrombosis, we analyzed the time to 90% occlusion in wild-type and HS1- and P2Y1-deficient mice (Figure 5C-F). Wild-type and P2Y1-deficient mice were used for standardization, because the in vivo thrombosis data have been well characterized in each of the mice.26,–28 P2Y1-deficient mice consistently failed to occlude during a 30-minute time period (Figure 5E), whereas the wild-type mice formed a stable thrombus in 7 minutes (Figure 5C). In the HS1-null mice, a prolonged time to occlusion of 10 minutes was observed, but the thrombus embolized 3 minutes later, which indicates a failure to form a stable thrombus over a 30-minute time period (Figure 5D). Thus, the HS1-deficient mice were characterized with the formation of unstable thrombi associated with embolization and an average time to occlusion of 22 minutes (Figure 5F). The impaired thrombus formation seen between the wild-type and the 2 genetically deficient mice is statistically significant (P < .01) (Figure 4F). Collectively, the impaired in vivo thrombosis data obtained with the HS1-null mice support our hypothesis that HS1 regulates platelet activation.
HS1 deficiency adversely compromises ex vivo platelet function
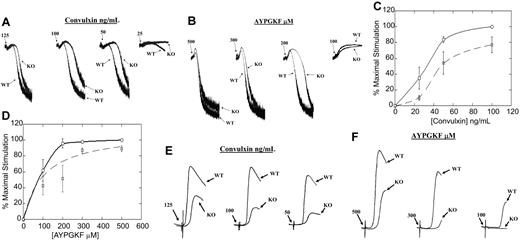
Results of the experiments described above showed that the lack of HS1 protein resulted in a bleeding diathesis along with prolonged thrombus and unstable clot formation in vivo. Hence, we examined the importance of HS1 in GPVI-mediated platelet activation. Stimulation with 25, 50, 100, and 125 ng/mL convulxin all showed impaired aggregation in HS1-null mouse platelets compared with the wild-type littermates. Low-dose convulxin (25 ng/mL) caused no aggregation and little shape change in HS1-null mouse platelets, whereas it produced significant shape change and detectable aggregation from platelets of wild-type littermates. Intermediate concentrations of 50 and 100 ng/mL convulxin produced a rightward shift that solely exhibited shape change in HS1-deficient platelets, compared with mild aggregation exhibited in wild-type littermates. This result indicates that the time taken for maximal aggregation is higher in the absence of HS1. Similarly, higher concentrations of convulxin (125 ng/mL) also produced a prolonged shape change and increased time to maximal aggregation in the HS1-deficient mouse platelets compared with those of their wild-type littermates (Figure 6A).
Effect of HS1 deficiency on ex vivo platelet functional responses. (A,B) Washed platelets from wild-type (WT) and HS1-null (KO) mice were stimulated with different concentrations of convulxin (A) or AYPGKF (B), and their aggregations were measured and compared. (C,D) Washed platelets from wild-type and HS1-null mouse platelets were stimulated with different concentrations of convulxin (C) or AYPGKF (D) and labeled with the JON/A αIIbβ3 activation-dependent antibody. Binding of the antibody was measured by flow cytometry and graphed (normalized to maximal stimulation). Wild-type mouse platelets are indicated by the solid line, and the HS1-null mouse platelets are indicated by the dashed line. A 2-way ANOVA was used to calculate the statistical significance for the difference in the dose-response curve (P< .05). Error bars indicate standard error. (E,F) Washed platelets from wild-type and HS1-null mice were stimulated with different concentrations of convulxin (E) and AYPGKF (F), and their dense-granule secretion was measured. The data are representative of at least 3 different experiments.
Effect of HS1 deficiency on ex vivo platelet functional responses. (A,B) Washed platelets from wild-type (WT) and HS1-null (KO) mice were stimulated with different concentrations of convulxin (A) or AYPGKF (B), and their aggregations were measured and compared. (C,D) Washed platelets from wild-type and HS1-null mouse platelets were stimulated with different concentrations of convulxin (C) or AYPGKF (D) and labeled with the JON/A αIIbβ3 activation-dependent antibody. Binding of the antibody was measured by flow cytometry and graphed (normalized to maximal stimulation). Wild-type mouse platelets are indicated by the solid line, and the HS1-null mouse platelets are indicated by the dashed line. A 2-way ANOVA was used to calculate the statistical significance for the difference in the dose-response curve (P< .05). Error bars indicate standard error. (E,F) Washed platelets from wild-type and HS1-null mice were stimulated with different concentrations of convulxin (E) and AYPGKF (F), and their dense-granule secretion was measured. The data are representative of at least 3 different experiments.
In vivo studies with GPVI- and FcRγ-deficient mice have shown no defect in bleeding times or in vivo thrombosis formation.29 The data described above, therefore, suggest that HS1 has a functional affect in multiple pathways; hence, we examined the functional importance of the HS1 in ex vivo PAR-mediated platelet-aggregation assays. Stimulation with 100, 200, 300, and 500 μM AYPGKF all resulted in impaired aggregation in HS1-null mouse platelets compared with those of wild-type littermates. Low-dose AYPGKF (100 μM) resulted in shape change in both HS1-null mouse platelets and their wild-type littermates. Moderate concentrations of 200 and 300 μM AYPGKF produced a rightward shift that exhibited prolonged shape change and increased time to maximal aggregation in HS1-deficient platelets compared with those of the wild-type littermates. Maximal concentrations of AYPGKF (500 μM) produced a slight prolongation in shape change and increased time to maximal aggregation in the HS1-deficient mouse platelets compared with those of the wild-type littermates (Figure 6B). To our knowledge, these results indicate for the first time that HS1 is involved in PAR-mediated αIIbβ3 activation.
Prolonged shape change is a characteristic of reduced activation of the αIIbβ3 integrin. Therefore, we examined both shape change and αIIbβ3 activation in wild-type and HS1-deficient littermates. Wild-type and HS1-null mice were stimulated at varying doses of convulxin in the presence of EDTA to prevent aggregation. It was interesting to note that the rate of shape change did not vary significantly between the 2 types of mice (data not shown). We next studied αIIbβ3 activation using the JON/A activation–specific antibody. Wild-type and HS1-knockout mouse platelets were stimulated with increasing concentrations of convulxin or AYPGKF and labeled with the JON/A–fluorescein isothiocyanate conjugated antibody. The platelets were analyzed by flow cytometry, and the amount of antibody bound to activated αIIbβ3 was calculated and normalized to maximal stimulation. A 2-way analysis of variance (ANOVA) indicated that the concentration-response curve acquired from αIIbβ3 activation was significantly lower in the HS1-null mice (P < .05) (Figure 6C,D). These results suggest that HS1 contributes to the inside-out signaling pathways that lead to αIIbβ3 activation.
Integrin activation observed by aggregometry and flow cytometry does not show a severe impairment of HS1-null mouse platelets. Because dense-granule release contributes to thrombus formation, we examined whether there was a role for HS1 in platelet secretion. Impaired secretion in HS1-null mouse platelets was observed compared with wild-type littermates after stimulation with 50, 100, and 125 ng/mL convulxin (Figure 6E). Low-dose convulxin caused no secretion in the HS1-null mouse platelets (data not shown). Similarly, stimulation with varying doses of AYPGKF (100, 300, and 500 μM) resulted in reduced secretion in HS1-null mouse platelets compared with those of wild-type littermates (Figure 6F). The data discussed above suggest that impaired platelet activation may be caused by an overall inhibition in platelet response.
We studied signaling downstream of GPVI to evaluate the effect of HS1 deficiency on the signaling cascade. As described previously, HS1 associates with PI3K, a downstream effector of GPVI signaling. Therefore, we examined Akt/PKB, a known mediator of aggregation and secretion and a downstream signaling molecule in the PI3K pathway. Convulxin-induced Akt phosphorylation was severely impaired in the HS1-null mouse platelets (Figure 7A). However, PAR-4–mediated Akt phosphorylation was inhibited less drastically in HS1-null mouse platelets compared with wild-type littermate mouse platelets (Figure 7B). Total PKCδ from the same gel was used as a loading control to ensure a lack of bias in lane loading and Western blot analysis. The difference in Akt phosphorylation downstream of GPVI stimulation further supports that HS1 has a role in inside-out platelet activation.
Effect of HS1 deficiency on agonist-induced Akt phosphorylation. Washed platelets from wild-type and HS1-deficient mice were stimulated with either convulxin (A) or AYPGKF (B) for 1 minute, and Western blot analysis of serine-phosphorylated Akt (Ser473) was performed on total cell lysates. PKCδ was used as a lane-loading control. The data are representative of at least 3 different experiments.
Effect of HS1 deficiency on agonist-induced Akt phosphorylation. Washed platelets from wild-type and HS1-deficient mice were stimulated with either convulxin (A) or AYPGKF (B) for 1 minute, and Western blot analysis of serine-phosphorylated Akt (Ser473) was performed on total cell lysates. PKCδ was used as a lane-loading control. The data are representative of at least 3 different experiments.
Discussion
Collagen-dependent activation through the GPVI receptor is an important signaling pathway that mediates many of the important functional responses in platelets, including αIIbβ3 activation, secretion, and adhesion to collagen. After vascular injury, adhesion of platelets to collagen in the subendothelium leads to autocoid secretion through the GPVI pathway. ITAM phosphorylation by Src kinases results in Syk phosphorylation, PLCγ2 phosphorylation, the activation of PKC, a rise in intracellular calcium (which leads to dense-granule secretion), and thromboxane generation. Similar signaling pathways, however, also mediate PKC activation and an increase in intracellular calcium mobilization downstream of the antigen receptors on B and T lymphocytes.30,31 HS1, a protein expressed exclusively in hematopoietic cells, has been found to be important in B- and T-cell proliferative and apoptotic responses. HS1 has been shown to be tyrosine phosphorylated and associated with both Syk- and Src-family kinases in B and T lymphocytes.14,32 The similarity in signaling between platelets and B and T cells led us to investigate whether HS1 has a functionally significant role in platelet activation.
Our results indicate that HS1 is phosphorylated downstream of GPVI activation and depends on the nonreceptor Src-family tyrosine kinases. Because we used aspirin-treated and washed platelets, we eliminated the generation of thromboxane A2 and its contribution to platelet activation. We investigated HS1 phosphorylation, independent of ADP secretion, using P2Y-receptor antagonists and have deduced that ADP secretion plays no obvious role in HS1 phosphorylation. The studies with PP1 and PP2 demonstrated that HS1 phosphorylation depends on the activity of Src-family kinases. Inhibition of Src-family kinases prevents phosphorylation of the tyrosine residues on the FcRγ chain, which renders Syk inactive. As a result, Syk-mediated primary phosphorylation of HS1 is blocked. Brunati et al19 recently showed that Src inhibition allows for some phosphorylation of HS1 downstream of thrombin activation. However, thrombin activates through G-protein–coupled receptors, which do not require Src for initiation of the signaling cascade. These data indicate that there are distinct pathways for HS1 phosphorylation downstream of both PAR and GPVI activation.
We found that HS1-null mice exhibit a bleeding phenotype along with prolonged thrombus and unstable clot formation in vivo compared with that of their wild-type littermates. However, it has been shown that in vivo thrombus formation is not impaired in mice that lack the GPVI or FcRγ chain.29 This indicates that the HS1 protein is involved in multiple pathways that cause the prolonged bleeding time and impaired thrombus formation in vivo.
We investigated the HS1 protein using wild-type and HS1-null mouse platelets to identify a possible functional role for HS1 in the platelet system. Using aggregation assays, we found that the lack of HS1 results in a rightward shift in the collagen convulxin- and PAR-4–dependent activation of platelets. The rightward shift in the concentration-response curve was explored further using flow-cytometric analysis of αIIbβ3 activation with the activation-specific JON/A antibody. Our results provided additional evidence of significantly impaired αIIbβ3 activation in response to convulxin and AYPGKF stimulation in HS1-deficient platelets, which indicates that HS1 may contribute to potentiation of platelet activation through multiple pathways.
It has been shown that HS1 phosphorylation leads to the translocation of the molecule to the membrane in platelets19 and in immature B lymphocytes.32 Thus, HS1 may act as a scaffolding protein and bring other signaling molecules in close proximity to each other to potentiate the platelet response. This paradigm is consistent with the observation that HS1 association with tyrosine-phosphorylated proteins increases after stimulation with GPVI agonists (Figure 1).
It has been hypothesized that there is a second adaptor protein in the GPVI signaling complex aside from LAT that could bring PLCγ2, Vav1/3, and SLP76 together. Gomez et al25 recently showed that HS1 associates with Vav1, PLCγ1, and SLP76 at the immune synapse in T cells. We hypothesize that HS1 may also associate with these proteins in GPVI signaling after translocation to the membrane, allowing full platelet activation. Thus, the reduced platelet activation by GPVI agonists in HS1- or LAT-deficient mice could be caused by a lack of such signaling complex formation.
PI3K plays an important role in PLCγ mobilization to the membrane and Akt phosphorylation in platelet GPVI signaling.33 PI3K inhibition has been shown to diminish p-selectin expression, aggregation, and Akt phosphorylation.34,35 Recent studies with Jurkat cells indicated that HS1 associates with PI3K.25 Our studies demonstrate HS1 association with the regulatory subunit, p85, and the catalytic subunit, p110, of PI3Ks in response to convulxin and AYPGKF in platelets. Treatment with pan-PI3K inhibitors wortmannin and LY294002 results in impaired platelet responses to agonist and protection against occlusive thrombus formation.36,37 Wortmannin is the more potent inhibitor and affects all type I PI3K isoforms and the type II PI3K-C2β isoform,37 which results in protection against thrombus formation and impaired platelet activation. Studies with platelets that lacked the p85α regulatory subunit showed impaired GPVI-induced platelet activation, including platelet aggregation and Akt phosphorylation.38 However, thrombin-induced platelet aggregation was not impaired in these p85α-null mice.38 Similarly, the p110δ-knockout mice exhibit impaired platelet function in response to collagen-related peptide activation but not to ADP, thromboxane A2, or thrombin.39 Mice that are deficient in the isoform p110γ fail to form a stable occlusion in response to the FeCl3-induced carotid-injury model and demonstrate impaired platelet function in response to ADP.34,40 The HS1-knockout mice have a mild impairment of in vivo and ex vivo platelet function possibly because of the association of HS1 with more than one PI3K isoform (Figure 3). Given the differences in the platelet responses to GPVI agonists and G-protein–coupled-receptor agonists in platelets from PI3K-subunit–null mice, it is plausible that the association between HS1 and PI3K subunits is greater after GPVI stimulation, which translates into diminished Akt phosphorylation downstream of GPVI stimulation in HS1-null mouse platelets.
Our results show that HS1-deficient mice exhibit a decrease in Akt phosphorylation, impaired dense-granule secretion, and impaired αIIbβ3 activation in response to convulxin and AYPGKF. Akt has also been implicated in fibrinogen-receptor activation20,41 and secretion. HS1 association with PI3K may be important in modulating a subsequent pathway such as Akt, which results in αIIbβ3 activation and dense-granule secretion. Akt might be the key signaling molecule downstream of both GPVI and PARs that is regulated by HS1.
In conclusion, our results show that GPVI stimulation leads to the phosphorylation of HS1 in a Src-family kinase–dependent manner. Furthermore, HS1 activation may potentiate GPVI signaling downstream of Syk activation. We demonstrate for the first time, to our knowledge, that HS1 associates with PI3K in platelets and that HS1-null mice have impaired Akt phosphorylation and secretion and αIIbβ3 activation. Consequently, HS1 is a functionally important signaling molecule that is involved in multiple pathways in the activation of platelets and in vivo thrombus formation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institutes of Health (NIH) research grants HL60683, HL80444, and HL81322 (to S.P.K.). B.N.K. and R.T.D. are supported by NIH Training grant in Thrombosis T32 HL07777.
National Institutes of Health
Authorship
Contribution: B.N.K. designed and performed research, collected and analyzed data, and wrote the paper; R.T.D. designed experiments, performed research, and collected and analyzed data; S.R.M. designed and performed experiments, and collected and analyzed data; S.K. analyzed data; T.J.S. designed and performed experiments; L.F.B. contributed analytical tools; J.L.D. analyzed data, contributed analytical tools, and provided reagents; D.K. generated and provided mice; and S.P.K. provided overall supervision of the project, analyzed data, and designed experiments.
B.N.K. and R.T.D. contributed equally to this manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satya P. Kunapuli, Temple University, Department of Physiology, Room 217 MRB, 3420 N Broad St, Philadelphia, PA 19140; e-mail:spk@temple.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal