The regulation of plasmin generation on cell surfaces is of critical importance in the control of vascular homeostasis. Cell-derived microparticles participate in the dissemination of biological activities. However, their capacity to promote plasmin generation has not been documented. In this study, we show that endothelial microparticles (EMPs) from tumor necrosis factor α (TNFα)–stimulated endothelial cells served as a surface for the generation of plasmin. The generation of plasmin involved expression of urokinase-type plasminogen activator (uPA) and its receptor (uPAR) at the surface of EMPs and was further increased by their ability to bind exogenous uPA on uPAR. Plasminogen was activated at the surface of EMPs in a dose-dependent, saturable, and specific manner as indicated by the inhibition of plasmin formation by ϵ-amino-caproic acid (ϵ-ACA) and carboxypeptidase B. EMP-induced plasmin generation affects tube formation mediated by endothelial progenitor cells. However, low amounts of EMPs increased tube formation, whereas higher concentrations inhibited it. Prevention of these effects by inhibitors of either uPA or plasmin underscore the key role of EMP-induced plasmin generation. In conclusion, we demonstrated that EMPs act as vectors supporting efficient plasmin generation and dissemination, a new pathway in the regulation of endothelial proteolytic activities with potential involvement in inflammation, angiogenesis, and atherosclerosis.
Introduction
Microparticles (MPs) are vesicles resulting from the blebbing of the cellular membrane of most activated or apoptotic cells.1 These microvesicles have been described in various cellular models and in different pathological conditions as reliable hallmarks of cell damage.2 Because they convey various bioactive effectors originating from the parent cells, MPs may exhibit a spectrum of biological activities: they regulate endothelial or blood cell functions, participate in inflammatory responses or angiogenesis, and propagate biological responses involved in hemostatic balance.3 We previously reported the capacity of endothelial cells to release microparticles after inflammatory stimulation and the presence of increased levels of circulating endothelial microparticles (EMPs) in patients with thrombotic disorders.4 Since this initial report, elevated levels of EMPs have been documented in various pathological conditions including coronary syndromes,5 renal failure,6 diabetes,7 antiphospholipid syndrome,8 thrombotic thrombocytopenic purpura,9 and sickle cell disease,10 in which they reflect endothelial dysfunction and are associated with a poor clinical outcome.
EMPs provide procoagulant phospholipid surfaces for the assembly and activation of coagulation factors, mainly through phosphatidylserine translocation to the exoplasmic leaflet as a result of membrane remodeling. Their involvement in thrombin generation also results from their capacity to harbor, deliver, or induce tissue factor activity.11,–13 However, a more complex contribution to the hemostatic balance is suggested by their expression of thrombomodulin, tissue factor pathway inhibitor, and endothelial protein C receptor, thus providing a possible antithrombotic counterbalance.14,15
Another key regulator of the vascular homeostasis is the plasminogen activation system. Plasminogen activation is mediated by 2 serine proteases: tissue-type plasminogen activator (tPA), which is mainly implicated in fibrinolysis, and urokinase-type plasminogen activator (uPA), which is critically involved in pericellular proteolysis due to its high affinity cell-surface receptor uPAR.16 Plasmin generation induced by uPA and subsequent activation of matrix metalloproteinases (MMPs) promote cell migration through interstitial matrix and participate in processes such as tissue remodeling, cancer invasion, and angiogenesis.17,–19 Importantly, we have shown that uncontrolled plasminogen activation can have deleterious consequences by inducing cell detachment and apoptosis.20,21 The regulation of plasmin generation at the endothelial surface is therefore of critical importance in the control of vascular homeostasis.
Because MPs convey protein and functional systems expressed by the parent cell, we hypothesized that EMPs may serve as active surfaces for interaction with plasminogen and plasmin formation, a hitherto undescribed function. The objective of this study was therefore to analyze the capacity of EMPs to bind and convert plasminogen into active plasmin and to define the impact of plasmin generation on EMP capacity to regulate angiogenic responses mediated by endothelial progenitor cells (EPCs) in vitro.
Materials and methods
Preparation of plasminogen and plasminogen-free fetal calf serum
Plasminogen was purified by lysine-affinity chromatography and molecular sieving as reported previously.22 Plasminogen-free fetal calf serum (FCS) was obtained by 3 cycles of affinity adsorption on lysine-Sepharose (4 FCS vol/1 gel vol) and was subsequently checked and found to contain less than 0.1 nM plasmin(ogen) activity.
Cell culture
The human microvascular endothelial cell line (HMEC-1), obtained from Dr Ades23 (Centers for Disease Control, Atlanta, GA), was cultured in MCDB 131 medium (Invitrogen Life Technologies, Cergy Pontoise, France) supplemented with 10% MP-free FCS, 10 ng/mL human recombinant epithermal growth factor (Upstate Cell Signaling Solutions, Lake Placid, NY), and 1 μg/mL hydrocortisone (Sigma, St Quentin Fallavier, France). Human vein endothelial cells (HUVECs) were obtained by collagenase digestion as previously described,24 cultured into 0.2% gelatin-coated flasks in EGM2 medium and used at passage 2. Human saphenous vein endothelial cells were purchased from Clonetics (Grand Island, NY) and cultured into 0.2% gelatin-coated flasks in EGM2-MV medium and used at passage 6.
Generation, harvesting, and flow cytometry enumeration of EMPs
For most experiments, EMPs were from HMEC-1 origin, as this cell line is well characterized and considered representative of the microcirculation, the core of the vascular tree.23,25 EMPs were purified from culture medium conditioned by subconfluent HMEC-1 stimulated for 48 hours with 100 ng/mL TNF-α (PeproTech, Rocky Hill, NJ) as previously described, with minor modifications.4 Culture supernatants from flasks were collected and cleared from detached cells or large cell fragments by centrifugation at 4300g for 5 minutes. The supernatants were then centrifuged at 20 000g for 120 minutes at 4°C. Pelleted EMPs were washed 2 times and resuspended in phosphate-buffered saline (PBS). The absence of residual TNF-α in this EMP sample was verified using an enzyme-linked immunosorbent assay (ELISA) assay (R&D Systems, Minneapolis, MN). Aliquots of 10 μL EMP suspension, 1/100 diluted, were labeled using fluorescein isothiocyanate (FITC)–conjugated annexin V (Abcys, Paris, France), and EMPs were enumerated by flow cytometry as previously described.26 The same protocol was used to obtain EMPs from quiescent HMEC-1, saphenous endothelial cells, and endothelial progenitor-derived cells (EPDCs).
Isolation and culture of endothelial progenitor cells from cord blood
Human umbilical cord blood samples (30-50 mL) were collected from donors, in compliance with French legislation, in a sterile tube containing heparin (200 UI/mL). Mononuclear cells (MNCs) were isolated by density gradient centrifugation. Briefly, blood was diluted 1:1 in PBS containing 2 mM ethylenediaminetetraacetic (PBS/EDTA) and layered over lymphocyte separation medium (Eurobio, Les Ulis, France). After a 30-minute centrifugation at 400g, MNCs were washed 3 times in PBS/EDTA. Cord blood MNCs were preplated in RPMI/10% FCS for 24 hours in plastic flasks. Nonadherent cells were plated onto 0.2% gelatin-coated 24-well plates (5.106 cells per well) and maintained in endothelial basal medium-2 (EBM-2) supplemented with EGM-2 SingleQuots (EGM-2 medium; Clonetics, Walkersville, MD). The medium was changed every 4 days. The appearance of well-circumscribed colonies with a cobblestone morphology was monitored daily. For expansion of EPDCs, colonies were trypsinized and cells were replated on a 6-well plate (passage 1). Subsequently, confluent cells were trypsinized and replated in T75 flasks for further passages. Cells were maintained under standard conditions (humidified atmosphere, 5% CO2, 37°C).
Progenitor endothelial-cell tube formation in matrigel
Flat-bottom 96-well plates were precoated with 1:1 mixture of cold matrigel basement membrane (10 mg/mL, BD Biosciences, Bedford, MA) and RMPI-10% FCS medium. After 45 minutes of polymerization at 37°C, EPDCs were plated at 2.105 cells/well in RPMI/10% FCS with increasing concentrations of EMP from TNF-α–stimulated HMEC-1 or an identical volume of the supernatant from the last EMP washing. To test the role of FCS plasminogen in this system, native FCS was replaced by plasminogen-free FCS supplemented or not with 1 μM human plasminogen. For inhibition experiments, EMPs were pre-incubated with either an anti-uPA antibody (394O, American Diagnostica, Greenwich, CT, 25 μg/mL) or an irrelevant control IgG1 (25 μg/mL) or with serine-protease inhibitors (aprotinine, 100 kui/mL or α2-antiplasmin, 1 μM). After 24 hours, capillary tube formation was evaluated by measuring the number of polygons formed in a well under an inverted light microscope (Olympus) at 400 × magnification. This time point was chosen following previous kinetics experiments indicating that it is optimum to analyze tube formation in the presence of EMPs. All experiments were performed in triplicate.
EMP immobilization
A physico-chemical adsorption principle was used to immobilize negatively charged EMPs on a polycation surface. For that purpose, round-bottom PVC plates were activated with 25 μg/mL poly-L-lysine, and various concentrations of EMP in PBS were then incubated overnight at 4°C with the activated surface. The plate was then washed, and the immobilized EMPs were tested.
Plasmin generation test
In round-bottom 96-well PVC plates, various concentrations of EMPs, either in suspension in PBS supplemented with 0.8% bovine serum albumin (PBSA) or immobilized on poly-L-lysine–coated plates, were incubated with 50 μL of 1 μM plasminogen and 0.75 mM of a chromogenic substrate selective for plasmin (methyl-malonyl)-hydroxypropylarginine-p-nitroanilide (CBS0065; Stago, Asnière, France). An identical volume of supernatant from the last EMP washing was used as control. In order to determine the Michaëlis constant of plasmin generation by EMPs, a fixed number (2.105) of EMPs and varying concentrations of plasminogen (0 to 5 μM) were used. Kinetics of plasmin generation were followed during 9 hours in a multiwell plate counter (MX5000, Dynex) at 37°C by measuring the change in absorbance at 405 nm produced by the release of p-nitroaniline. In the case of activation experiments performed on immobilized EMPs, the plate was washed with PBSA to eliminate unbound reactants, and the plasmin bound to the immobilized EMPs was detected by adding 50 μL/well of 0.75 mM CBS0065 and measuring the change in absorbance at 405 nm. When indicated, the following inhibitors were pre-incubated with the EMPs: 1 μg/mL goat antihuman tPA (Biopool, Uppsala, Sweden), 50 μg/mL mouse antihuman uPA (394O), 50 μg/mL rabbit anti-αvβ3 (gift of J.C. Lissitzky), 100 μg/mL anti–α-enolase (gift of R. Lopez Alemany), and the respective irrelevant control IgGs (Biocytex, Marseille, France); the uPA inhibitor, amiloride, was used at 100 μM and carboxypeptidase B (CpB) at 50 μg/mL, final concentrations. In separate experiments, the inhibitors α2-antiplasmin, aprotinin, and ϵ-amino-caproic acid (ϵ-ACA) were added to the plasminogen activation solutions at 100 nM, 100 kiu/mL and 0.1 M final concentrations, respectively.
Characterization of uPA and uPAR in cell and EMP extracts
HMEC-1 and pelleted MPs were lysed in 100 mM tris(hydroxymethyl)aminomethane (Tris)–HCl buffer pH 8.1, containing 0.5% Triton X-100 and supplemented with complete protease inhibitor mixture (Roche Diagnostic, Mannheim, Germany) except for zymography samples. Lysates were clarified and protein concentrations were determined using the BCA kit (Pierce, Rockford, IL). uPA and uPAR total antigen levels were assayed by ELISA (894 and 893 IMUBIND ELISA kits, American Diagnostica) according to the manufacturer's instruction. The results were expressed as ng of uPA or uPAR per mg of total proteins. For zymography, protein extracts (5 μg), molecular weight markers, and purified scuPA were electrophoresed under nonreducing conditions in 10% sodium dodecyl sulfate (SDS)–polyacrylamide gel copolymerised with 1 mg/mL α-casein from bovine milk (Sigma) and 20 μg/mL human plasminogen. After electrophoresis, SDS was eluted from the gel by washing for 1 hour in 2.5% Triton X-100 buffer. The gels were then incubated for 40 hours at 37°C in 50 mM Tris-HCl buffer, pH 8, containing 5 mM CaCl2, 138 mM NaCl, and 0.03% Brij 35. Zymograms were developed by staining with Coomassie Brillant Blue Dye and destained to reveal clear bands of casein lysis, indicative of enzymatic activity. Samples run on casein gels without plasminogen or gels incubated in lysis buffer supplemented with 1 mM amiloride (Sigma) served as controls of uPA activity. For Western blot analysis, protein extracts and purified human uPAR (American Diagnostica) used as positive control (20 μg/lane) were separated under nonreducing conditions by 8% SDS-polyacrylamide gel and electrotransferred onto a nitrocellulose membrane. After blocking, the membranes were incubated overnight at 4°C with rabbit antihuman uPAR (399R, American Diagnostica, 0.5 μg/mL) antibodies, followed by the horseradish peroxidase–conjugated secondary antibodies. Immunocomplexes were visualized with the Supersignal West Pico chemiluminescence kit (Pierce).
Immunoelectron microscopy of EMPs
For uPA/uPAR immunogold labeling of EMPs, droplets of MP isolated from TNFα-stimulated HMEC-1 were applied to 300 mesh nickel Formvar-carbon-coated grids (Agar Scientific, Essex, United Kingdom) for 10 minutes. Samples were immunolabeled with either anti-uPA (3689, American Diagnostica, 10 μg/mL) or anti-uPAR (3932, American Diagnostica, 10 μg/mL) antibodies for 1 hour, washed with PBS, and then reacted with the 15-nm beads gold-labeled secondary antibody (BBInternational, Cardiff, United Kingdom) for 1 hour. Grids were rinsed and negatively stained with 0.3% phosphotungstic acid (pH 7) before observation with a JEOL 1220 electron microscope. Specificity of immunolabeling was determined in comparison to results obtained with an irrelevant control antibody or with gold-labeled secondary antibody alone.
Flow cytometry for uPA and uPAR on EMPs
EMPs (10 μL) were labeled with either anti-uPA (394O, 25 μg/mL) or anti-uPAR (3932, 25 μg/mL) antibodies for 1 hour, then FITC-labeled secondary antibody was added and incubated for 30 minutes before samples were analyzed on a Cytomics FC500* flow cytometer (Beckman Coulter, Fullerton, CA). MPs were analyzed according to the FSC/SSC characteristic in a gate with the upper limit defined by beads of 0.9 μm and the lower limit defined by 0.3 μm. Specificity of labeling was determined in comparison to results obtained with an irrelevant control antibody or with the secondary antibody alone.
Binding of uPA to EMPs
A fixed concentration of EMPs was immobilized on poly-L-lysine–coated 96-well plates. Increasing concentrations (0 to 18.5 nM) of sc-uPA (a kind gift of Dr H.R. Lijnen, University of Leuven) were incubated during 1 hour at 37°C with the immobilized microparticles in the presence of 4 μg/mL of poly-L-lysine in order to eliminate nonspecific binding of sc-uPA to the poly-L-lysine surface. To verify the specificity of the binding, a fixed concentration of native sc-uPA (0.1 nM) was mixed with a 10 molar excess (1 nM) of a recombinant inactive form “rsc-uPA Ile 159→Gly” (H.R. Lijnen, University of Leuven, Leuven, Belgium). The native sc-uPA, specifically bound to EMPs, was detected by measuring plasmin generation as described above.
Statistical analysis
Data are expressed as mean ± standard deviation. Statistical analysis was performed with Prizm software (GraphPad Software, San Diego, CA) and with KaleidaGraph software (Synergy Software, Reading, PA). Significant differences were determined using nonparametric Mann-Whitney test. A P value less than 0.05 was considered significant.
Results
EMPs are able to activate plasminogen into plasmin
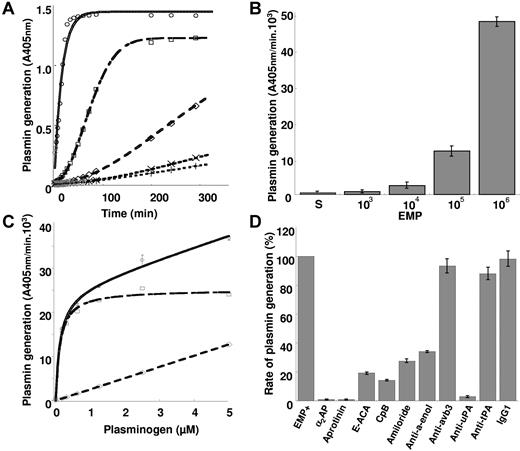
To investigate the ability of EMPs to generate plasmin, the microparticles were incubated with plasminogen and a plasmin-selective chromogenic substrate. As shown in Figure 1A, plasmin generation occurred as a function of time and was proportional to the number of EMPs. At 106 EMP/well the plasmin generation rate was 47.6 ± 1.1 A405nmx10−3/min, 53-fold higher than at 103 EMP/well (0.9 ± 0.3 A405nmx10−3/min) (Figure 1B). At identical EMP concentrations (2 105/50 μL) comparable results were obtained using EMP derived from quiescent or TNFα-stimulated HMEC-1. In contrast, the level of plasmin generated by EMPs was shown to vary according to their endothelial cell origin (Table 1). Thus, a more pronounced activity was produced by HMEC-1–derived MPs as compared with MPs of macrovascular origin (saphenous vein, HUVEC), whereas intermediate values were obtained for EPDC-derived MPs. Plasminogen incubated with EMPs was activated in a dose-dependent, saturable, and specific manner (Figure 1C, Km = 0.122 μM, Vmax = 25.2 A405 nm/min 103). As expected, plasmin activity was completely blocked in the presence of α2-antiplasmin or aprotinin (Figure 1D).
EMPs are able to activate plasminogen into plasmin. (A) Plot of plasmin generated versus time at varying EMP amounts per 50 μL/well (○ = 106; □ = 105; ◇ = 104; x = 103; +‖= control without EMPs) and fixed final concentrations of plasminogen (1 μM) and a plasmin-selective chromogenic substrate (0.75 mM). Representative graph of 4 independent experiments. (B) Similar experiment as in panel A expressed as change in absorbance at 405 nm per minute versus EMP amount per well (S: EMP last washing supernatant used as control). (C) Plasmin generated at varying plasminogen concentrations (0-5 μM) and a fixed amount (2.105/50 uL) of EMPs was detected with a chromogenic substrate as in panel A. Raw data (○) were fitted to the Michaelis-Menten equation allowing calculation of nonspecific activity (◇) and a Km = 0.122 μM for specific plamin generation (□). (D) Effect of various inhibitors on the generation of plasmin by EMPs (2.105/50 μL) at 0.5 μM plasminogen (α2AP = α2-antiplasmin; ϵ-ACA = ϵ-amino-caproïc acid; CPB = carboxypeptidase B; antibodies to uPA and tPA αvβ3 and α-enolase as compared with an isotype control IgG1). Results are the mean ± SD of 3 independent experiments.
EMPs are able to activate plasminogen into plasmin. (A) Plot of plasmin generated versus time at varying EMP amounts per 50 μL/well (○ = 106; □ = 105; ◇ = 104; x = 103; +‖= control without EMPs) and fixed final concentrations of plasminogen (1 μM) and a plasmin-selective chromogenic substrate (0.75 mM). Representative graph of 4 independent experiments. (B) Similar experiment as in panel A expressed as change in absorbance at 405 nm per minute versus EMP amount per well (S: EMP last washing supernatant used as control). (C) Plasmin generated at varying plasminogen concentrations (0-5 μM) and a fixed amount (2.105/50 uL) of EMPs was detected with a chromogenic substrate as in panel A. Raw data (○) were fitted to the Michaelis-Menten equation allowing calculation of nonspecific activity (◇) and a Km = 0.122 μM for specific plamin generation (□). (D) Effect of various inhibitors on the generation of plasmin by EMPs (2.105/50 μL) at 0.5 μM plasminogen (α2AP = α2-antiplasmin; ϵ-ACA = ϵ-amino-caproïc acid; CPB = carboxypeptidase B; antibodies to uPA and tPA αvβ3 and α-enolase as compared with an isotype control IgG1). Results are the mean ± SD of 3 independent experiments.
Plasmin generation by endothelial microparticles
| Source from which EMPs were derived . | Plasmin generation, mean % plus or minus SD . |
|---|---|
| TNFα-stimulated HMEC-1 | 100 |
| Quiescent HMEC-1 | 98.0 ± 6.3 |
| EPDC | 27.7 ± 5.5 |
| Saphenous vein endothelial cells | 13.0 ± 2.6 |
| HUVEC | 7.6 ± 3.3 |
| Source from which EMPs were derived . | Plasmin generation, mean % plus or minus SD . |
|---|---|
| TNFα-stimulated HMEC-1 | 100 |
| Quiescent HMEC-1 | 98.0 ± 6.3 |
| EPDC | 27.7 ± 5.5 |
| Saphenous vein endothelial cells | 13.0 ± 2.6 |
| HUVEC | 7.6 ± 3.3 |
2 × 105/well.
Plasminogen is activated by uPA at the surface of EMPs
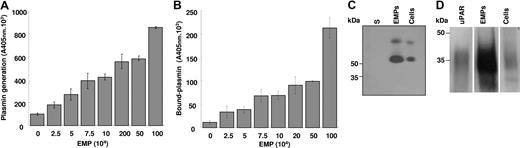
Supernatants from EMP washing failed to generate plasmin, ruling out the contribution of soluble factors and suggesting that the activation of plasminogen was dependent on factors associated with the EMP surface. To test this hypothesis, plasminogen activation experiments were performed on immobilized microparticles. In this system, plasmin generation was a function of the number of immobilized EMPs (Figure 2A). Plasmin was already detected on immobilized EMPs at a concentration of 25 000 EMP/well and increased progressively in a dose-dependent manner. At the end of the activation experiments the immobilized EMPs were washed, and bound plasmin was detected using a plasmin-selective chromogenic substrate. The amount of EMP-bound plasmin increased as a function of the number of immobilized EMPs (Figure 2B). Since some plasmin was released into the medium during its generation, the amount bound after washing was lower than the total amount of plasmin formed.
Plasminogen is activated at the surface of EMPs by uPA. (A,B) A variable number of EMPs immobilized on poly-L-lysine surfaces were incubated with fixed concentrations of plasminogen (0.5 μM) and the plasmin-selective chromogenic substrate (0.75 mM). The graph in panel A shows an increase in the formation of plasmin as a function of the number of immobilized microparticles. Unbound reagents were then washed off and the chromogenic substrate added to detect plasmin that remained bound to the immobilized EMPs; the graph (B) shows an increase in bound plasmin as a function of the number of immobilized EMPs. Representative graphs (mean ± SD) of 3 independent experiments. (C,D) Protein extracts from HMEC-1 and its derived EMPs were processed for zymography (panel C) to detect plasminogen activator activity (5 μg protein/lane; S: EMP last washing supernatant) and for immunoblot (panel D) using rabbit antibodies against uPAR and purified uPAR as reference (20 μg protein/lane). The ∼50 kDa and upper bands in panel C were inhibited by antibodies to urokinase (not shown). A space has been inserted to indicate where a gel lane was cut. These gels came from different experiments as indicated by the space between the gels.
Plasminogen is activated at the surface of EMPs by uPA. (A,B) A variable number of EMPs immobilized on poly-L-lysine surfaces were incubated with fixed concentrations of plasminogen (0.5 μM) and the plasmin-selective chromogenic substrate (0.75 mM). The graph in panel A shows an increase in the formation of plasmin as a function of the number of immobilized microparticles. Unbound reagents were then washed off and the chromogenic substrate added to detect plasmin that remained bound to the immobilized EMPs; the graph (B) shows an increase in bound plasmin as a function of the number of immobilized EMPs. Representative graphs (mean ± SD) of 3 independent experiments. (C,D) Protein extracts from HMEC-1 and its derived EMPs were processed for zymography (panel C) to detect plasminogen activator activity (5 μg protein/lane; S: EMP last washing supernatant) and for immunoblot (panel D) using rabbit antibodies against uPAR and purified uPAR as reference (20 μg protein/lane). The ∼50 kDa and upper bands in panel C were inhibited by antibodies to urokinase (not shown). A space has been inserted to indicate where a gel lane was cut. These gels came from different experiments as indicated by the space between the gels.
In parallel experiments using EMP in suspension, plasmin generation was inhibited by the lysine analog ϵ-ACA, suggesting a lysine-dependent mechanism for plasminogen binding and activation that was confirmed by abrogation of plasmin formation upon cleavage of C-terminal lysine residues on EMPs by CpB (Figure 1D). Among proteins bearing C-terminal lysine residues, α-enolase was identified as a major binding protein as indicated by 65% inhibition of plasmin formation with the specific antibody 11G1 (Figure 1D). The integrin αvβ3 that may influence plasminogen activation, mainly through interactions with the uPA/uPAR system, was shown not to be involved in plasmin generation by EMPs as indicated by the absence of effect of a specific neutralizing antibody (Figure 1D).
The uPA/uPAR complex is present on EMPs
The value determined for the apparent Km of plasminogen activation on EMPs was within the range determined for the uPA/uPAR system on endothelial cells and a variety of other cell types.27,28 The plasminogen activator conveyed by EMPs was identified as uPA as indicated by the inhibition of plasmin generation by (1) amiloride, a specific inhibitor of uPA, and by (2) an antibody specific for uPA (> 95% inhibition) (Figure 1D). By contrast, an antibody directed against tPA had no significant effect on plasmin generation. The presence of uPA activity on EMPs was further confirmed by a lytic band on casein zymography corresponding to the molecular mass of uPA (Figure 2C), which was also absent in gels into which amiloride (1 mM) was incorporated (data not shown). Comparison of the lytic bands from the EMPs and the parent cells, normalized for protein content, showed a 3- to 4-fold increase of uPA in the EMPs (representative experiment in Figure 2C). Similarly, when uPA antigen in EMPs and cells were measured by ELISA, a 3-fold increase in uPA was found in the EMPs (23.9 ± 12.6 versus 8.3 ± 3.17 ng uPA/mg protein, respectively, P < .05). UPAR was identified in EMPs and cell lysates by Western blot and was found to comigrate with mammalian uPAR. As for uPA, the EMPs had more uPAR than the cells when normalized for equivalent total protein concentrations (Figure 2D). The uPAR antigen level measured by ELISA was 4-fold higher in the EMPs than in the parent HMEC-1 (57.7 ± 14.54 versus 4.4 ± 1.17 ng/mg protein, respectively, P < .05). The presence of uPA or uPAR on the EMP surface also was analyzed by flow cytometry (Figure 3A,B). The shift of fluorescence histogram after specific labeling of EMPs with uPA and uPAR antibody compared with the irrelevant staining confirmed the presence of uPA and uPAR at the EMP surface.
Identification of uPA, uPAR, and uPA/uPAR complex on EMP. (A,B) Measured fluorescence intensity by flow cytometry analysis of EMPs with anti-uPA (A) and anti-uPAR (B) antibodies (black) and the corresponding isotype controls (gray). (C) Representative images of immunogold labeling of uPA (top) and uPAR (bottom) analysis on EMPs by transmission electron microscopy. Slides were viewed with a JEOL 1220 transmission electron microscope (JEOL, Tokyo, Japan). Images were acquired using a MegaView III camera (Soft Imaging System, Munster, Germany) and were processed with Analysis software (Soft Imaging System) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA). The dimension of the bars indicate the relative small size of these EMPs, and the clusters of 15-nm gold particles (black dots) indicate the presence of uPA and uPAR at the surface of EMPs. (D) Main graph: isotherm of the binding of varying amounts of scuPA incubated with EMPs immobilized on a poly-L-lysine surface. The amount of bound scuPA was detected by its ability to activate plasminogen using a chromogenic substrate selective for plasmin. Data fitted to the Langmuir equation as indicated in “Materials and methods” allowed calculation of a dissociation constant, Kd = 0.1 nM, for the interaction of scuPA with its receptor. Representative graph (mean ± SD) of 3 independent experiments. Inset: specificity of the binding of native scuPA is demonstrated by its inhibition with a modified recombinant form of scuPA (r-scuPA, ILe159→Gly) that bind to its receptor but cannot be activated. The bars represent the mean (± SD) of 3 independent experiments.
Identification of uPA, uPAR, and uPA/uPAR complex on EMP. (A,B) Measured fluorescence intensity by flow cytometry analysis of EMPs with anti-uPA (A) and anti-uPAR (B) antibodies (black) and the corresponding isotype controls (gray). (C) Representative images of immunogold labeling of uPA (top) and uPAR (bottom) analysis on EMPs by transmission electron microscopy. Slides were viewed with a JEOL 1220 transmission electron microscope (JEOL, Tokyo, Japan). Images were acquired using a MegaView III camera (Soft Imaging System, Munster, Germany) and were processed with Analysis software (Soft Imaging System) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA). The dimension of the bars indicate the relative small size of these EMPs, and the clusters of 15-nm gold particles (black dots) indicate the presence of uPA and uPAR at the surface of EMPs. (D) Main graph: isotherm of the binding of varying amounts of scuPA incubated with EMPs immobilized on a poly-L-lysine surface. The amount of bound scuPA was detected by its ability to activate plasminogen using a chromogenic substrate selective for plasmin. Data fitted to the Langmuir equation as indicated in “Materials and methods” allowed calculation of a dissociation constant, Kd = 0.1 nM, for the interaction of scuPA with its receptor. Representative graph (mean ± SD) of 3 independent experiments. Inset: specificity of the binding of native scuPA is demonstrated by its inhibition with a modified recombinant form of scuPA (r-scuPA, ILe159→Gly) that bind to its receptor but cannot be activated. The bars represent the mean (± SD) of 3 independent experiments.
EMPs also were analyzed by electron microscopy. Negative staining of the pellet revealed intact irregular-shaped EMPs ranging from 100 to 500 nm in size. Molecules of uPA and uPAR were detected on the outer surface after immunogold labeling with specific antibodies (Figure 3C). No labeling was observed in experiments using control antibodies.
Binding of sc-uPA to EMP-uPAR
Since the amount of uPAR measured by ELISA was higher on EMPs than on cells, we tested the capacity of immobilized EMPs to bind exogenous scuPA specifically. A range of concentrations of scuPA was incubated with the immobilized EMPs, washed, and then tested in a plasminogen activation assay. Figure 3D shows that scuPA binding was dose-dependent, saturable, and specific, as indicated by the inhibition of the binding by a modified form of scuPA that resists activation (Figure 3D, insert). Analysis of raw data with the Langmuir equation for single-site binding allowed calculation of a very low dissociation constant (Kd = 0.1 nM), indicating a very high affinity of scuPA for its EMP anchored receptor, in agreement with previous published data for the uPA/uPAR system.28 Since elution of intrinsic uPA could not be performed before the binding experiments, these results indicate that the amount of uPA originally bound is relatively small compared with the high uPAR binding capacity of EMPs.
EMPs affect tube formation by endothelial progenitor cells in vitro
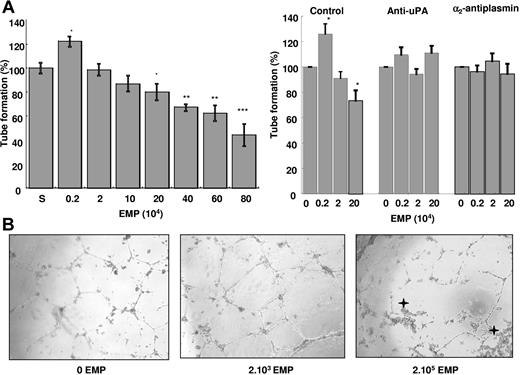
EPCs were supplemented with increasing amounts of EMPs (2.103 to 8.105), and tube formation in matrigel was evaluated using the supernatant from the last EMP washing as control (100% tube formation). As shown on Figure 4A, a biphasic effect was observed: at low EMP concentrations (≤ 2.103/well), polygon number and tube formation increased by 20% over control (P = .012), whereas higher amounts of EMP (≥ 2.105 EMPs/well) had the reverse effect. Thus, tube formation decreased progressively with the increase in the number of EMPs reaching 55% decrease below control (P < .001) at 8.105 EMPs/well. Concomitant with the decrease in tube formation, morphologic cell changes were observed (rounded cells and retracted cell clusters) (Figure 4B). The moderate pro-angiogenic effect of MP seems to be less potent as compared with vascular endothelial growth factor (VEGF) (see the Supplemental Materials link at the top of the online article available on the Blood website).
EMPs affect the angiogenic properties of endothelial progenitors in vitro: effects on tube formation in matrigel. EPDC in RPMI/10% FCS and varying amounts of EMPs were plated on matrigel, and tube formation was evaluated after 24 hours of incubation. (A) Biphasic effect of EMPs on tube formation: at low EMP concentrations (≤ 2.103/well), polygon number and tube formation increased by 20% (P = .012, n = 12) over control (S: EMP last washing supernatant). However, higher amounts of EMPs (≥ 2.105 EMP/well) had the reverse effect and progressively decreased the number of tubes to 50% at 8.105 EMP/well. Stars indicate significant changes (*P < .05, **P < .01, ***P < .001). Results are the mean (± SD) of 12 independent experiments. (B) Inverted light microscope representative images (400 × magnification) showing EPDC tube formation in matrigel in the absence and presence of 2.103 or 2.105 EMPs per well. Asterisks indicate retracted cell clusters. Slides were viewed with an inverted Nikon light microscope (Nikon, Melville, NY) using an Olympus lens (40×/0.60; Olympus, Tokyo, Japan). Images were acquired using a Nikon model DXM 1200F camera and were processed with Lucia G software (both from Nikon) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA). (C) Experiments were performed in the presence of inhibitors of either plasmin formation (anti-uPA antibody) or activity (α2-antiplasmin, α2-AP). The biphasic effect of EMPs on tube formation (left bar panel) was abolished by the anti-uPA antibody (middle) and by α2-AP (right). Asterisks indicate significant changes (P < .05). Results are the mean (± SD) of 3 independent experiments.
EMPs affect the angiogenic properties of endothelial progenitors in vitro: effects on tube formation in matrigel. EPDC in RPMI/10% FCS and varying amounts of EMPs were plated on matrigel, and tube formation was evaluated after 24 hours of incubation. (A) Biphasic effect of EMPs on tube formation: at low EMP concentrations (≤ 2.103/well), polygon number and tube formation increased by 20% (P = .012, n = 12) over control (S: EMP last washing supernatant). However, higher amounts of EMPs (≥ 2.105 EMP/well) had the reverse effect and progressively decreased the number of tubes to 50% at 8.105 EMP/well. Stars indicate significant changes (*P < .05, **P < .01, ***P < .001). Results are the mean (± SD) of 12 independent experiments. (B) Inverted light microscope representative images (400 × magnification) showing EPDC tube formation in matrigel in the absence and presence of 2.103 or 2.105 EMPs per well. Asterisks indicate retracted cell clusters. Slides were viewed with an inverted Nikon light microscope (Nikon, Melville, NY) using an Olympus lens (40×/0.60; Olympus, Tokyo, Japan). Images were acquired using a Nikon model DXM 1200F camera and were processed with Lucia G software (both from Nikon) and Adobe Photoshop version 7.0 software (Adobe Systems, San Jose, CA). (C) Experiments were performed in the presence of inhibitors of either plasmin formation (anti-uPA antibody) or activity (α2-antiplasmin, α2-AP). The biphasic effect of EMPs on tube formation (left bar panel) was abolished by the anti-uPA antibody (middle) and by α2-AP (right). Asterisks indicate significant changes (P < .05). Results are the mean (± SD) of 3 independent experiments.
Plasmin is involved in the EMP effects on tube formation
An anti-uPA antibody and α2-antiplasmin were used to determine whether the observed effects of EMPs on EPC tube formation were related to plasmin generation. As illustrated in Figure 4C, in the presence of these inhibitors, EMPs had no more effect on tube formation.
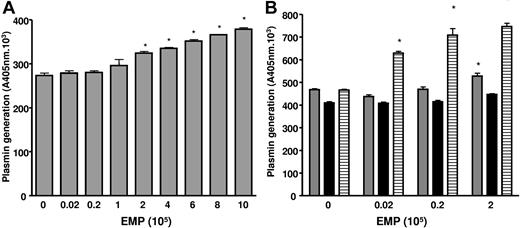
We also considered the presence of plasmin in the matrigel medium, because the gel itself and the culture medium (RPMI/10% FCS) contain plasminogen,29 and the EMPs are a source of uPA. The plasmin chromogenic substrate was added with the EMPs in matrigel, allowing detection of increased plasmin generation from 105 EMP (P < .001) at 2 hours of incubation (Figure 5A). The contribution of FCS plasminogen to plasmin formation was estimated using plasminogen-free FCS (Figure 5B). Under these conditions plasmin generation was moderately reduced (−11.8%, P = .001) compared with native FCS and was not influenced by the amount of EMPs added, suggesting the presence of a basal plasmin generation in the matrigel. The addition of plasminogen to plasminogen-free FCS restored plasmin formation to the level observed with native FCS in the absence of EMPs and was again importantly increased as a function of the number of added EMPs (more than 75% increase at 2.105 EMP/well, Figure 5B). These data confirmed the role of matrigel and plasminogen from FCS in plasmin generation in the absence of EMPs, and the strong potential capacity of EMPs to enhance plasmin generation.
EMPs are involved in plasmin generation in matrigel. (A) Plasmin generation in matrigel is dependent on the amount of EMPs. The chromogenic substrate CBS0065 was added to the matrigel with the EMPs, and under these conditions an increase in plasmin formation was detected from 105 EMPs (P < .001) after 2 hours of incubation. Asterisks indicate significant changes (P < .05). Results are the mean (± SD) of 3 independent experiments. (B) Plasmin generation in matrigel at different EMP amounts is dependent on the supply of plasminogen by FCS. The graph shows plasmin activity detected after 3 hours of incubation ▒, medium with 10% FCS; ▓, medium with plasminogen-free FCS; ▤, medium with plasminogen-free FCS supplemented with 1 μM plasminogen). Activity in the absence of EMPs may represent low levels of plasmin and/or limited amounts of activators in matrigel. Asterisks indicate significant changes (P < .05). Results are the mean (± SD) of 3 independent experiments.
EMPs are involved in plasmin generation in matrigel. (A) Plasmin generation in matrigel is dependent on the amount of EMPs. The chromogenic substrate CBS0065 was added to the matrigel with the EMPs, and under these conditions an increase in plasmin formation was detected from 105 EMPs (P < .001) after 2 hours of incubation. Asterisks indicate significant changes (P < .05). Results are the mean (± SD) of 3 independent experiments. (B) Plasmin generation in matrigel at different EMP amounts is dependent on the supply of plasminogen by FCS. The graph shows plasmin activity detected after 3 hours of incubation ▒, medium with 10% FCS; ▓, medium with plasminogen-free FCS; ▤, medium with plasminogen-free FCS supplemented with 1 μM plasminogen). Activity in the absence of EMPs may represent low levels of plasmin and/or limited amounts of activators in matrigel. Asterisks indicate significant changes (P < .05). Results are the mean (± SD) of 3 independent experiments.
Altogether, these data indicate that the uPA/uPAR system of EMPs is responsible for plasmin generation and that plasmin proteolytic activity is involved in the EMP effects on tube formation by EPCs.
Discussion
The plasminogen activation system plays a pivotal role in maintaining vascular patency and facilitating cell migration and angiogenesis. Binding of plasminogen to fibrin or the cell surface is of critical importance to regulate and target the proteolytic activity. Although the presence of uPAR on endothelial microparticles previously has been described,30 its functional consequences, to our knowledge, have not been investigated as yet. The present study is the first demonstration that EMPs provide a catalytic surface for the conversion of plasminogen into plasmin by expressing uPA and uPAR. Plasmin generation was EMP surface-dependent and could be augmented by binding of exogenous uPA to EMP-uPAR. As a result, EMPs were shown to modulate the angiogenic responses of endothelial progenitors in vitro.
The generation of plasmin at the EMP surface was supported by inhibition experiments using the lysine analog ϵ-ACA and CpB. The inhibitory effect of ϵ-ACA, which occupies the lysine-binding site of plasminogen kringles 1 and 4, indicated lysine-dependent binding of plasminogen to the activation surface. The inhibition of plasmin formation by CpB confirmed that plasminogen activation was dependent on its cell-surface binding to C-terminal lysine residues. This mechanism was further confirmed by inhibition of plasmin generation with a monoclonal antibody directed against α-enolase, a major plasminogen binding protein on cell surfaces.31 Taken together, these data demonstrated that EMPs bind plasminogen and provide a catalytic surface for plasmin generation. The kinetics of plasminogen activation by the EMPs were similar to those described for cells expressing uPA and uPAR.27,28,32 Accordingly, ELISA and zymography experiments revealed that EMPs express uPA and uPAR. Part of this uPA and uPAR expression was located on the EMP surface as evidenced by electron microscopy and flow cytometry.
The major contribution of EMP-bound uPA to the plasmin generation was further demonstrated by the following observations: (1) plasmin generation was blocked by the uPA inhibitor amiloride and by a uPA-neutralizing monoclonal antibody, and (2) tPA-dependent lytic activity was absent on zymograms, and neutralizing tPA antibodies did not affect plasmin generation.
EMPs also were shown to bind exogenous sc-uPA. Both the affinity of sc-uPA binding and the competitive effect of a recombinant form of uPA (rsc-uPA Ile159→Gly), consistent with uPA/uPAR interaction, demonstrate that the EMP surface bears uPAR molecules devoid of uPA, allowing the specific binding of exogeneous uPA. These data, together with the expression of uPAR in excess of uPA, suggest that plasmin generation by EMPs can be amplified by uPA transferred from the local environment to the EMP surface. This mechanism may have implications in tumor angiogenesis, where uPA secretion is promoted. Thus, MPs from tumor cells and EMPs could participate in amplification of proteolytic processes.33
Several reports have demonstrated that cell surface–bound plasmin is responsible for pericellular proteolysis and matrix degradation as compared with the soluble form.27,34 This efficient activity results from protection against inactivation by physiological inhibitors, a direct contact with its matrix substrates, and the existence of amplification loops for plasminogen activation.16 Interestingly, plasmin once formed remains partly bound to the EMP surface. Collectively, these data show that EMPs act as vectors of efficient plasmin generation.
This proteolytic activity is not the hallmark of cell activation by TNFα since it was similarly observed on EMPs derived from quiescent endothelial cells. The substantial differences in plasmin generation observed among various endothelial cell types is consistent with the heterogeneous expression level of uPA/uPAR system along the vascular tree.35
Vascular homeostasis is the result of an equilibrium between injury and capacity for repair.3 Regeneration of damaged endothelium recently has been shown to involve not only angiogenesis but also vasculogenesis mediated by endothelial progenitors, both processes being largely dependent on proteolytic activities.35 Thus, the capacity of EMPs to behave as vector of proteolytic activities raises the possibility that EMPs may modulate EPC-mediated repair processes. Consistently, apoptotic bodies from HUVECs have been shown to stimulate differentiation of endothelial progenitor, thereby representing a potential signaling pathway linking damaged cells to progenitors in the facilitation of the repair process.36 In our study, coculture experiments showed that EMPs affect EPC angiogenesis in matrigel in a concentration-dependent manner. While low amounts of EMPs increased tube formation, higher concentrations inhibited it. This dual effect involved EMP-associated plasmin since (i) in our coculture model, EMPs retained the capacity to increase plasmin concentration by activating the plasminogen of the culture medium, and (ii) EMP effect was abrogated in the presence of α2-antiplasmin and a uPA-blocking antibody. The proangiogenic effect of EMPs is consistent with plasmin-associated proteolytic activity that favors cell migration via extracellular matrix processing and direct interaction with αvβ3 integrin.19 The latter, however, is not involved in the process of plasmin generation (Figure 1D). Although this proangiogenic effect was observed with EMPs present in a low number, plasmin was readily detected, indicating that the local concentration of plasminogen was sufficient to ensure efficient plasmin generation. Other mechanisms also may be involved. MPs from HUVECs stimulated with growth factors have been shown to stimulate angiogenic properties of mature endothelial cells, and this effect was related to their content in matrix metalloproteinases.37 Consistently, we observed an increase in MMP-2 and MMP-9 activities in condition media of matrigel experiments in the presence of low dose of EMPs (data not shown). Plasmin also may affect angiogenesis indirectly through activation of these MMPs.18
In our study, high concentrations of EMPs produced high amounts of plasmin and a dose-dependent decrease in tube formation by EPCs. This inhibitory effect is in line with the role of an excessive plasmin generation leading to extracellular matrix degradation, alteration of cell anchorage, and apoptosis.20,38 Morphological changes in EPCs cocultured with high EMP amounts were indeed observed: accumulation of round and retracted cells evoking cell detachment, an effect that precedes apoptotic cell death.21 Protective and noxious effects of plasmin formation also have been observed in a cellular model of amyloid β degradation, suggesting that a similar effect may occur in vivo.39 Our data add to the observations of Mezentsev et al (2005) that reported a major contribution of oxidative stress in the impairment of angiogenic behavior of mature endothelial cells.40 Although, in this study no proangiogenic effect at low doses of EMPs was observed, this discrepancy may be related to the specific properties of endothelial progenitors. EPCs have been reported to display increased sensitivity to proangiogenic stimulation and reduced sensitivity to oxidative stress compared with mature endothelial cells.41 Hence, the magnitude of an MP angiogenic signal may be influenced not only by MP cellular origin and concentration but also by the nature of the target cells.
In conclusion, we have identified cell-derived MPs as new actors in the plasminogen activation system, an undescribed facet of the multiple biological processes involving EMPs in the control of vascular homeostasis. The role of EMP-bound plasmin in pathological settings involving inflammation, atherosclerosis, angiogenesis, and tumor growth remains to be investigated. The high concentration of MPs reported in atherosclerotic plaques suggests that EMP-induced plasmin generation could participate in the modulation of cell apoptosis/angiogenesis balance, influencing plaque vulnerability. In stroke, released EMPs could be actors linking inflammation, cell damage, and dysregulated repair processes.42
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Agence Nationale pour la Recherche: Programme ANR-05-PCOD-024.
We gratefully acknowledge the contribution of Dr H.R. Lijnen (Catholic University of Leuven, Leuven, Belgium) for providing scu-PA and rsc-uPA Ile 159→Gly; Dr R. Lopez Alemany (Institu Recerca Oncologica, Barcelona, Spain) for providing the monoclonal antibody 11G1 directed against α-enolase; and Dr J.C. Lissitzky (UFR Pharmacie, Marseille, France) for providing the anti-αvβ3 antibody. We also thank Prof Marc Gamerre and his staff (Department of Gynecology and Obstetrics, CHU Conception, Marseille, France) for collection of cord blood samples.
E.A.-C. and his team are funded by an Inserm-Avenir/Lower-Normandy Council grant.
Authorship
Contribution: R.L. performed research (plasmin generation by microparticles, angiogenesis assays), collected data, analysed and interpreted data, and participated in manuscript drafting; F.S. contributed to isolation and culture of endothelial progenitors and participated in data interpretation and in manuscript drafting; A.M. performed research (characterisation of uPA-uPAR expression by microparticles); A.B. performed research (flow cytometry experiments, statistical analysis); R.P. performed research (zymography); H.B. contributed to electronical microscopy experiments; S.R. performed research (flow cytometry experiments); E.L. performed research (zymography); L.C.-J. contributed to research design and microparticle production; L.P. performed research (plasminogen preparations and fibrin-agar zymography); V.G. contributed to study design and editing of the manuscript; E.A.-C. designed research, contributed analytical tools, analyzed data, and participated in manuscript drafting; and F.D.-G. designed research, analyzed data, and participated in manuscript drafting.
F.D.-G. and E.A.-C. share senior authorship for this study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Dignat-George, Inserm UMR 608, UFR de Pharmacie, 27 Bd Jean Moulin, 13385-cdx Marseille 5, France; e-mail:dignat@pharmacie.univ-mrs.fr; or Eduardo Angles-Cano, Inserm/Cyceron/Université de Caen, Bd Henri Becquerel, 14074, Caen, France; e-mail: angles@cyceron.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal