Nuclear factor-κB (NF-κB) plays a crucial role in B-cell and lymphoid organ development. Here, we studied the consequences of constitutive, signal-independent activation of the alternative NF-κB pathway for the splenic marginal zone (MZ). In contrast to nfkb2−/− mice, which lack both p100 and p52, mice that lack only the inhibitory p100 precursor but still express the p52 subunit of NF-κB2 (p100−/−) had markedly elevated MZ B-cell numbers. Both cell-intrinsic mechanisms and increased stromal expression of vascular cell adhesion molecule-1 (VCAM-1) contributed to the accumulation of MZ B cells in p100−/− spleens. While migration of p100−/− MZ B cells toward the lysophospholipid sphingosine-1 phosphate (S1P) was not affected, CXCL13-stimulated chemotaxis was impaired, correlating with reduced migration of MZ B cells into follicles in response to lipopolysaccharide (LPS). Strikingly, p100 deficiency resulted in the absence of a normal marginal sinus, strongly induced expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and glycosylated cell adhesion molecule-1 (GlyCAM-1), and the formation of nonfunctional ectopic high endothelial venule (HEV)–like structures in the red pulp. Thus, constitutive activation of the alternative NF-κB pathway favors MZ B-cell development and accumulation but leads to a disorganized spleen microarchitecture.
Introduction
The spleen plays an important role in the filtration of blood and the defense against pathogens. The blood-filtering system and the purge of old erythrocytes are largely confined to the splenic red pulp. The white pulp provides a spatial microenvironment that facilitates the interaction between pathogens and various cell populations required for an efficient immune response. Red and white pulp are separated by a highly ordered region termed the marginal zone (MZ).1,2 The MZ is characterized by the presence of MZ B cells as well as specialized macrophages with a unique array of scavenger molecules, such as marginal metallophilic macrophages (MMMs) at the inner side and MZ macrophages (MZMs) at the outer side of the MZ.3 Interestingly, both development and maintenance of the splenic MZ are crucially dependent on B cells because the MZ and its constituents disappear concurrently with the loss of B cells.4
In the spleen, newly formed/transitional stage 1 (NF/T1) B lymphocytes that have just left the bone marrow are believed to differentiate into T2 follicular precursors (T2-FPs), which may either differentiate into follicular B (FO B) cells or sequentially into T2 MZ B-cell precursors (T2-MZPs) and MZ B cells.5 Circulating FO B cells represent the majority of mature B cells that lodge in their specific area within the white pulp following CXCL13 chemokine gradients.6 B1 cells, which are enriched predominantly in the pleural and peritoneal cavities, share many phenotypic and functional characteristics with MZ B cells.7 MZ B cells, which harbor features clearly distinguished from FO B cells, fulfill initial antibody responses against bloodborne antigens together with B1 cells. Although MZ B cells can be induced to migrate into the white pulp, they are regarded as a relatively sessile population. Recently, mechanisms governing their lodging and retention in the MZ have been intensively studied. Signaling through receptors for lysophospholipid sphingosine-1 phosphate (S1P) is involved in the proper localization of MZ B cells in the MZ.8,9 After stimulation with lipopolysaccharide (LPS), MZ B cells down-regulate expression of S1P1 and S1P3 receptors and subsequently relocalize to B-cell follicles in a CXL13-dependent manner. Another mechanism for long-term retention of MZ B cells in the MZ is the interaction between integrins on B cells and integrin ligands expressed on splenic stromal cells, such as αLβ2 and α4β1 integrins, which bind to intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), respectively.10 In addition, it has been suggested that MZMs are involved in the proper localization of MZ B cells.11
Recirculating lymphocytes enter the spleen via marginal sinuses by extravasating a layer of mucosal addressin cellular adhesion molecule-1–positive (MAdCAM-1+) sinus-lining cells that form a barrier between MZ and white pulp.12 In contrast, entry of naive lymphocytes from the bloodstream into lymph nodes (LNs) and Peyer patches (PPs) occurs via specific postcapillary high endothelial venules (HEVs).13 HEVs facilitate L-selectin–mediated lymphocyte adherence and extravasation through expression of peripheral node addressin (PNAd), adhesion molecules, and chemokines.13 PNAd, a ligand for L-selectin and binding to the MECA-79 mAb, is generated by highly glycosylated sialomucins, including glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1) and CD34.14 The chemokine CCL21 is produced by HEV endothelial cells and is expressed by more than 80% of HEVs. HEV-specific gene expression appears to be regulated by lymphotoxin (LT) signaling and activation of the Nuclear factor-κB (NF-κB) pathway.15,16
The alternative pathway of NF-κB activation is triggered by receptors, such as lymphotoxin-β receptor (LTβR) or B-cell–activating factor receptor (BAFF-R). It is controlled through NF-κB–inducing kinase (NIK) and the IκB kinase (IKK) α subunit of the IKK complex. Phosphorylation of the inhibitory p100 precursor leads to its processing to the p52 form of NF-κB2 and nuclear translocation of p52-RelB heterodimers.17,,–20 Formation of the splenic MZ and generation of MZ B cells are critically dependent on the activation of p52-RelB.21,22 To address the consequences of constitutive, signal-independent activation of the alternative NF-κB signaling pathway, we have used p100-deficient knock-in mice (p100−/−) that still express the p52 subunit of NF-κB2,23 compared with nfkb2−/− knock-out mice that lack both p100 and p52.24,25 We show that p100 deficiency results in a marked increase of MZ B-cell numbers and their retention in the splenic MZ. Moreover, constitutive signaling through the alternative NF-κB pathway leads to the absence of a normal marginal sinus and the induction of ectopic HEV-like structures in the splenic red pulp. Thus, a tight control of the alternative NF-κB pathway and RelB activity is essential for normal B-cell development and organization of the splenic MZ.
Materials and methods
Mice
Generation of p100−/−, nfkb2−/−, and relB−/− mice has been described previously.23,24,26 All mice were crossed onto a C57BL/6 background for more than 10 generations. Reducing litter size and feeding wet food largely avoided early lethality of p100−/− mice during the first 3 weeks.23 All animals were analyzed between 17 and 20 days after birth. Bone marrow chimeras were generated as previously described.21 Briefly, donor bone marrow cells were isolated from femora of wild-type or p100−/− mice and injected (4–6 × 106 cells intravenously per mouse) into 2- to 3-month-old C57BL/6 recipients. Before injection, recipient mice were irradiated with 2 × 5.5 Gy (Gammacell 40 Exactor for a 3-hour interval; Nordion International, Vancouver, BC) and rested for 3 to 4 hours after the second irradiation. Chimeric mice were analyzed 6 to 8 weeks after reconstitution. For LPS treatment, wild-type and p100−/− mice were injected intraperitoneally with LPS (1 mg/kg in PBS, serotype O55:B5; Sigma, Taufkirchen, Germany), and spleens were analyzed 4 hours later. FITC-labeled heat-killed Escherichia coli bacteria (Invitrogen, Karlsruhe, Germany) were reconstituted in PBS at a concentration of 108/mL, and 50 μL were injected intravenously into 18-day-old p100−/− mice and littermate controls. After 15 minutes, mice were killed and spleens were removed and processed for immunofluorescence. All animals were housed and bred under standardized conditions with water and food ad libitum in the specific pathogen–free (SPF) mouse facility of the Leibniz-Institute for Age Research–Fritz-Lipmann-Institute.
Flow cytometry and immunohistochemistry
Splenocytes were isolated and red blood cells (RBCs) were lysed according to standard procedures. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). Immunohistochemistry was performed essentially as described.21 For details, see Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Cell culture, detection of apoptotic cells, B-cell migration, and adhesion assays
Apoptotic cells were detected by Annexin-V–FITC (BD Bioscience, Heidelberg, Germany). Briefly, 107 splenocytes were cultured in 1 mL media (RPMI, 10% FCS, 2 mM glutamine, 50 μM β-mercaptoethanol, 100 U/mL penicillin, and 100 μg/mL streptomycin) or stimulated with LPS (10 μg/mL; serotype O55:B5) or anti-CD40 mAb (10 μg/mL; clone 1C10). After 48 hours, cells were harvested and stained with the appropriate antibodies to discriminate MZ B (CD23−IgMhiCD21hi) and FO B cells (CD23+IgMloCD21int). Cells were washed, stained in Annexin-V solution for 15 minutes at room temperature, washed twice, and resuspended for flow cytometric analysis. Annexin-V+ cells were indicative of apoptosis. B-cell migration and adhesion assays were performed essentially as described (Document S1).10,27
QRT-PCR analysis
Total RNA isolation was performed following the manufacturer's instructions using NucleoSpin RNA II kit (Macherey & Nagel, Düren, Germany). Contamination of DNA was removed by DNase I treatment. Primers for quantitative real-time–polymerase chain reaction (QRT-PCR) with melting temperature (Tm) of 58–60°C were designed using Primer Express Software v2.0 (Applied Biosystems, Darmstadt, Germany). Triplicates were performed for all QRT-PCR reactions using reagents supplied by Quantace (Watford, United Kingdom) with an iCycler real-time PCR machine (Bio-Rad, Munich, Germany). Primer sequences are shown in Document S1.
Results
Characterization of B-cell subsets in p100−/− spleens
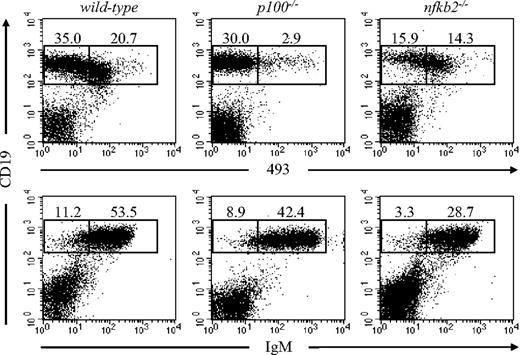
The splenic B-cell compartment mainly consists of immature, transitional, and mature B cells. To analyze the role of p100 in the splenic MZ, we first evaluated B-cell subsets in p100−/− spleens. Since p100−/− mice die 3 to 4 weeks after birth due to marked gastric hyperplasia,23 we analyzed 17- to 20-day-old animals. The CD19+493+ B-cell compartment containing immature and transitional B cells was reduced 7-fold in p100−/− spleens. In contrast, the mature B-cell compartment (CD19+493−) was less affected, which was further confirmed by the almost normal frequency of IgMlo mature B cells (Figure 1). B1 cells, which express the pan–T-cell surface antigen CD5 and are B220lo, represent a small portion of mature B cells in the spleen. No significant differences between wild-type and p100−/− B1 cells (B220loCD5lo) in the spleen were observed, although a clear reduction (3- to 4-fold) of peritoneal B1 cells was found in p100−/− mice (Figure S1). Importantly, these defects were restricted to mice lacking p100. In nfkb2−/− mice, which lack both p100 and p52, a preferential reduction in the splenic mature B-cell compartment was observed (Figure 1), whereas the frequency of B1 cells in the spleen and the peritoneal cavity was normal (Figure S1), indicating that the lack of both p52 and p100 affects mainly mainstream B2 rather than B1 cells. In contrast to B cells, we observed an increased percentage of T cells and myeloid cells in p100−/− spleens (Figure S2). The absolute number of T cells, however, remained unchanged due to the reduced number of total lymphocytes in the spleen. Together, these data indicate that the loss of the inhibitory p100 precursor affects predominantly the immature and transitional B-cell compartment in the spleen, and that the p52 subunit of NF-κB is required for these defects.
Reduced percentage of immature B cells in spleens from p100−/− mice. Splenocytes from 17- to 20-day-old wild-type, p100−/−, and nfkb2−/− mice were stained for CD19 and the early B-cell marker 493 (top row) or CD19 and IgM (bottom row) and analyzed by flow cytometry (lymphocyte gate). Numbers indicate percentages from indicated regions. Representative data from 3 to 4 experiments are shown.
Reduced percentage of immature B cells in spleens from p100−/− mice. Splenocytes from 17- to 20-day-old wild-type, p100−/−, and nfkb2−/− mice were stained for CD19 and the early B-cell marker 493 (top row) or CD19 and IgM (bottom row) and analyzed by flow cytometry (lymphocyte gate). Numbers indicate percentages from indicated regions. Representative data from 3 to 4 experiments are shown.
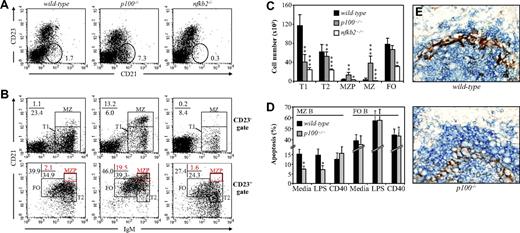
Flow cytometric analysis of p100−/− spleens revealed an increase of CD21hiCD23− MZ B cells (7.3% vs 1.7% in wild-type), which was specific for p100−/− mice since the combined inactivation of p52 and p100 in nfkb2−/− mice caused a considerable reduction of MZ B cell numbers (Figure 2A). Newly formed immature T1 B cells (IgMhiCD21−CD23−) differentiate into T2 B cells (IgMhiCD21+CD23+), which include presumed MZ precursor B cells (MZPBs; IgMhiCD21hiCD23+) that further develop into mature IgMhiCD21hiCD23− MZ B cells.5 The strong expansion of p100−/− MZ B cells (13.2% CD21hiIgMhi vs 1.1% in wild-type) was confirmed upon CD21 and IgM staining of CD23− splenocytes. In contrast, the percentage of CD23− T1 B cells (CD21−IgMhi) was reduced (6.0%) compared with wild-type littermates (23.4%) (Figure 2B; top panels). In the CD23+ population, no significant differences were observed between T2 (CD21+IgMhi) and FO (CD21intIgMlo) B cells from p100−/− and control mice. However, within the T2 population, a small subset of CD23+CD21hiIgMhi MZP B cells was expanded in the p100−/− spleens, indicating an increase of immediate precursors of MZ B cells (Figure 2B; bottom panels). Calculating absolute numbers of the different splenic subpopulations confirmed that loss of p100 results in a decrease of T1 B cells and an increase in both MZPBs and mature MZ B cells. This increase in MZ B-cell numbers was dependent on p52, since spleens from nfkb2−/− mice showed a reduction in all B-cell subpopulations, including MZ B cells (Figure 2B,C). The decrease of T1 B cells in p100−/− spleens was likely to originate from the bone marrow, since immature bone marrow CD19+IgMhi B cells, the immediate precursors of splenic T1 B cells, were 4-fold reduced compared with littermate controls23 (data not shown).
Mice lacking p100 have markedly increased numbers of MZ B cells. (A) Splenocytes from wild-type, p100−/−, and nfkb2−/− mice were stained for CD23 and CD21 and analyzed by flow cytometry (lymphocyte gate). Percentages indicate CD23−CD21+ MZ B cells. (B) CD23− (top row) and CD23+ (bottom row) splenocyte subpopulations from wild-type, p100−/−, and nfkb2−/− mice were analyzed for CD21 and IgM expression. Percentages of newly formed T1 B cells (CD23−CD21−IgMhi), T2 B cells (CD23+CD21+IgMhi), MZ B cells (CD23−CD21hiIgMhi), and FO B cells (CD23+CD21intIgMlo) are shown (black regions). Among the T2 B-cell subpopulation, percentages of presumed MZ precursor B cells (MZPB; CD23+CD21hiIgMhi) are indicated (red regions). Representative data from 3 to 4 experiments are shown. (C) Absolute numbers of B-cell subpopulations per 106 splenocytes from wild-type, p100−/−, and nfkb2−/− mice are shown. Error bars indicate standard deviation (SD) from at least 5 mice per genotype. (D) MZ B cells from p100−/− mice show better survival than wild-type controls. Splenocytes were cultured for 48 hours in media or stimulated with LPS or anti-CD40 mAb. Percentages of Annexin-V+ apoptotic cells in MZ and FO B-cell subpopulations are shown. Error bars indicate SD from 3 experiments. Significant differences are indicated (Student t test, *P < .05; **P < .01; ***P < .002). (E) Spleens from p100−/− mice exhibit an enlarged MZ B-cell population adjacent to MOMA-1+ macrophages. Spleen sections from age-matched wild-type and p100−/− mice were stained with anti-IgM for B cells (Vector Blue) and MOMA-1 for MMMs (DAB brown). Objective: × 40. Images were acquired through an AX70 Olympus microscope (Olympus, Hamburg, Germany) with a 40×/0.75 objective by an Olympus DP70 digital camera running analySISB Soft Imaging System software (Olympus) and processed with Adobe Photoshop 8.0 software (Adobe, San Jose, CA).
Mice lacking p100 have markedly increased numbers of MZ B cells. (A) Splenocytes from wild-type, p100−/−, and nfkb2−/− mice were stained for CD23 and CD21 and analyzed by flow cytometry (lymphocyte gate). Percentages indicate CD23−CD21+ MZ B cells. (B) CD23− (top row) and CD23+ (bottom row) splenocyte subpopulations from wild-type, p100−/−, and nfkb2−/− mice were analyzed for CD21 and IgM expression. Percentages of newly formed T1 B cells (CD23−CD21−IgMhi), T2 B cells (CD23+CD21+IgMhi), MZ B cells (CD23−CD21hiIgMhi), and FO B cells (CD23+CD21intIgMlo) are shown (black regions). Among the T2 B-cell subpopulation, percentages of presumed MZ precursor B cells (MZPB; CD23+CD21hiIgMhi) are indicated (red regions). Representative data from 3 to 4 experiments are shown. (C) Absolute numbers of B-cell subpopulations per 106 splenocytes from wild-type, p100−/−, and nfkb2−/− mice are shown. Error bars indicate standard deviation (SD) from at least 5 mice per genotype. (D) MZ B cells from p100−/− mice show better survival than wild-type controls. Splenocytes were cultured for 48 hours in media or stimulated with LPS or anti-CD40 mAb. Percentages of Annexin-V+ apoptotic cells in MZ and FO B-cell subpopulations are shown. Error bars indicate SD from 3 experiments. Significant differences are indicated (Student t test, *P < .05; **P < .01; ***P < .002). (E) Spleens from p100−/− mice exhibit an enlarged MZ B-cell population adjacent to MOMA-1+ macrophages. Spleen sections from age-matched wild-type and p100−/− mice were stained with anti-IgM for B cells (Vector Blue) and MOMA-1 for MMMs (DAB brown). Objective: × 40. Images were acquired through an AX70 Olympus microscope (Olympus, Hamburg, Germany) with a 40×/0.75 objective by an Olympus DP70 digital camera running analySISB Soft Imaging System software (Olympus) and processed with Adobe Photoshop 8.0 software (Adobe, San Jose, CA).
One possible explanation for the increased MZ B-cell numbers in p100−/− spleens is increased cell survival. Indeed, when p100−/− MZ B cells were analyzed for apoptosis, an almost 2-fold decrease in the percentage of Annexin-V+ cells was observed in unstimulated and LPS-stimulated cultures, but not in anti-CD40–stimulated cultures, compared with wild-type controls. Apoptosis in treated or untreated p100−/− FO B cells was comparable with wild-type littermates, and MZ B cells showed in general better survival than FO B cells (Figure 2D). Interestingly, lack of p100 also resulted in a mild increase in expression of CD40 and CD80, suggesting an even more pronounced “activated” phenotype28 than wild-type MZ B cells (Figure S3).
To directly assess MZ B-cell localization in situ, spleen sections were stained with antibodies directed against MMMs (anti–MOMA-1) and B cells (anti-IgM). In agreement with the flow cytometry data, p100−/− spleens exhibited an enlarged MZ B-cell population adjacent to MOMA-1+ macrophages (Figure 2E). Together, these results indicate that the constitutive activation of the alternative NF-κB signaling pathway due to the absence of the p100 inhibitor favors the expansion or accumulation of MZ B cells in the splenic MZ.
Accumulation of MZ B cells in p100−/− mice is cell intrinsic
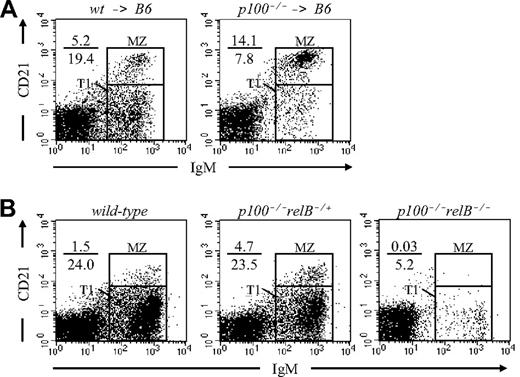
The accumulation of MZ B cells in p100−/− spleens could be due to either B-cell–autonomous defects or defects in the stromal microenvironment. To clarify this point, bone marrow reconstitution experiments were performed. After lethal irradiation, C57BL/6 recipients were reconstituted with either wild-type (wt → B6) or p100−/− (p100−/− → B6) bone marrow cells, and chimeras were analyzed 6 weeks after transplantation. In p100−/− → B6 chimeric spleens, the MZ B-cell population was clearly increased (3-fold) compared with wt → B6 controls (Figure 3A). A similar result was obtained in competitive mixed chimeras using CD45.1+ wild-type and CD45.2+p100−/− donors (Figure S4). Thus, lack of p100 results in a cell-autonomous expansion of MZ B cells in adult chimeric mice, although stromal factors may contribute as well.
Accumulation of MZ B cells in p100−/− mice is cell intrinsic. (A) Lethally irradiated C57BL/6 recipients were reconstituted with either wild-type (wt → B6) or p100−/− bone marrow cells (p100−/− → B6). At 6 weeks after transplantation, splenocytes from chimeras were stained for CD23, CD21, and IgM and analyzed for MZ B cells (CD23−CD21hiIgMhi). Only CD23− lymphocytes are shown. (B) MZ B-cell accumulation in p100−/− mice is dependent on relB. Splenocytes from wild-type, p100−/−relB−/+, and p100−/−relB−/− mice were analyzed for MZ B cells as described in panel A.
Accumulation of MZ B cells in p100−/− mice is cell intrinsic. (A) Lethally irradiated C57BL/6 recipients were reconstituted with either wild-type (wt → B6) or p100−/− bone marrow cells (p100−/− → B6). At 6 weeks after transplantation, splenocytes from chimeras were stained for CD23, CD21, and IgM and analyzed for MZ B cells (CD23−CD21hiIgMhi). Only CD23− lymphocytes are shown. (B) MZ B-cell accumulation in p100−/− mice is dependent on relB. Splenocytes from wild-type, p100−/−relB−/+, and p100−/−relB−/− mice were analyzed for MZ B cells as described in panel A.
Truncations in the carboxy-terminal region of the nfkb2 gene are associated with the development of lymphoid malignancies.29 We did not find obvious evidence for tumors in p100−/− → B6 chimeric mice, possibly due to the fact that they were killed already 6 to 8 weeks after bone marrow reconstitution.
MZ B-cell accumulation is partially reduced by deleting one relB allele in p100−/− mice
Mice lacking the inhibitory p100 precursor show increased NF-κB binding, consisting predominantly of p52-RelB heterodimers.23 To address whether RelB contributed to the accumulation of MZ B cells in p100−/− spleens, we generated p100−/−relB−/+ and p100−/−relB−/− mice. Flow cytometric analysis of 3-week-old mice revealed a partial reduction of MZ B-cell numbers in p100−/−relB−/+ spleens compared with p100−/−relB+/+ littermates. Mice lacking both p100 and RelB had an almost complete absence of MZ B cells, resembling the defect in relB−/− mice21 (Figure 3B). Collectively, these observations suggest that constitutive high levels of RelB in splenic p100−/− B-cell precursors favor the generation of MZ B cells, and that the lack of RelB cannot be compensated by other NF-κB family members, even in the absence of the p100 inhibitor.
Chemokine expression in p100−/− spleens
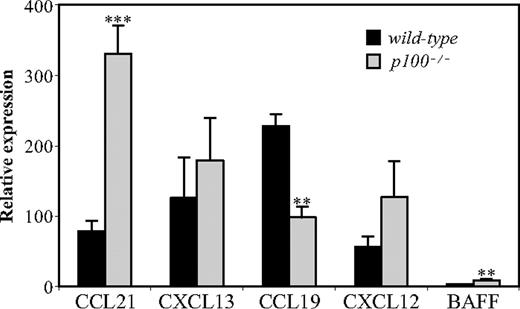
We have previously reported that expression of homing chemokines, which are crucial for a proper organization of the splenic microarchitecture, is reduced in mice lacking RelB.21,22 We therefore compared mRNA levels of CXCL12/SDF-1, CXCL13/BLC, CCL19/ELC, and CCL21/SLC in spleens from wild-type and p100−/− mice by QRT-PCR. While expression of CXCL13 and CXCL12 was not significantly increased, CCL21 mRNA levels were markedly up-regulated (4-fold) in the absence of the p100 inhibitor. We also observed increased expression of mRNA encoding BAFF, which was previously shown to be up-regulated upon LT signaling.30 Surprisingly, lack of p100 resulted in decreased (2-fold) mRNA levels of CCL19 (Figure 4), indicating that the related chemokines CCL19 and CCL21 are differentially regulated by p100/NF-κB2.
Deregulated chemokine expression in p100−/− spleens. Total RNA from wild-type and p100−/− spleens was analyzed for chemokine expression by QRT-PCR. Relative mRNA expression corrected for β-actin levels is shown. Error bars indicate SD from 3 to 4 experiments. Significant differences are indicated (Student t test, **P < .01; ***P < .002).
Deregulated chemokine expression in p100−/− spleens. Total RNA from wild-type and p100−/− spleens was analyzed for chemokine expression by QRT-PCR. Relative mRNA expression corrected for β-actin levels is shown. Error bars indicate SD from 3 to 4 experiments. Significant differences are indicated (Student t test, **P < .01; ***P < .002).
Impaired LPS-induced relocation of p100−/− MZ B cells
MZ B cells do not recirculate, but undergo migration to lymphoid follicles after exposure to bacterial products, such as LPS.31 To test whether this relocation was altered in the absence of p100, mice were injected with LPS, and spleens were examined by immunohistochemistry. Within 4 hours of LPS exposure, wild-type MZ B cells completely relocated into the white pulp, whereas a minimal MZ B-cell redistribution was observed in p100−/− spleens (Figure 5A). S1P receptor signaling regulates proper B-cell localization in the splenic MZ,8,9 and LPS-induced relocation of MZ B cells requires the chemokine CXCL13.10 We therefore examined B-cell chemotaxis to S1P and CXCL13 (Figure 5B). Both wild-type and p100−/− MZ B cells responded strongly to S1P, whereas migration of FO B cells was much weaker. In contrast, migration of p100−/− MZ B cells to CXCL13, and also to a lesser extent p100−/− FO B cells, was reduced compared with wild-type controls. Surface expression of the CXCL13 receptor, CXCR5, was indistinguishable between wild-type and p100−/− MZ B cells (data not shown).
Impaired LPS-induced relocation of p100−/− MZ B cells. (A) Wild-type or p100−/− mice were injected with PBS or LPS as indicated. After 4 hours, spleens were removed and frozen sections were stained for IgM (blue) and MOMA-1 (brown). Note that a significant number of B cells remained outside the MOMA-1+ ring in spleens from LPS-treated p100−/− mice. Images were acquired as in Figure 2E with a 20×/0.70 objective. (B) Impaired migration of p100−/− MZ B cells to CXCL13. B-cell chemotaxis to CXCL13 and S1P was analyzed in Transwell assays. Error bars indicate SD from 3 to 4 experiments. Significant differences are indicated (Student t test, **P < .01; ***P < .002).
Impaired LPS-induced relocation of p100−/− MZ B cells. (A) Wild-type or p100−/− mice were injected with PBS or LPS as indicated. After 4 hours, spleens were removed and frozen sections were stained for IgM (blue) and MOMA-1 (brown). Note that a significant number of B cells remained outside the MOMA-1+ ring in spleens from LPS-treated p100−/− mice. Images were acquired as in Figure 2E with a 20×/0.70 objective. (B) Impaired migration of p100−/− MZ B cells to CXCL13. B-cell chemotaxis to CXCL13 and S1P was analyzed in Transwell assays. Error bars indicate SD from 3 to 4 experiments. Significant differences are indicated (Student t test, **P < .01; ***P < .002).
Integrin-mediated adhesion of p100-deficient B cells
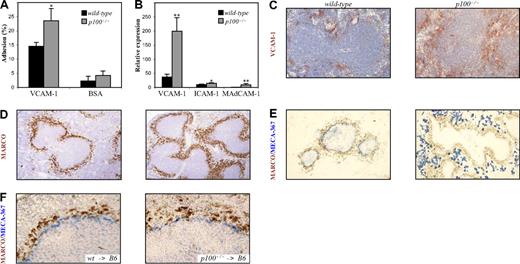
Analysis of surface integrin expression by flow cytometry revealed moderately increased levels of β1 on p100−/− compared with wild-type MZ B cells (Figure S3). When integrin-mediated adhesion to VCAM-1 was measured, we observed increased binding of MZ B cells from p100−/− mice compared with wild-type controls (Figure 6A). We next examined expression of NF-κB–regulated integrin ligands in spleens from p100−/− mice by QRT-PCR. While ICAM-1 mRNA levels were only slightly increased (1.5-fold), expression of VCAM-1 (5-fold) and MAdCAM-1 mRNA (9-fold) was markedly up-regulated in p100−/− compared with wild-type spleens (Figure 6B). The strong VCAM-1 expression in p100−/− mice was largely restricted to the MZ as shown by immunohistochemical staining of spleen sections (Figure 6C). Together, these results indicate that both decreased chemotaxis to CXCL13 and increased adhesion to VCAM-1 contribute to the retention of B cells in the MZ of p100−/− spleens and the impaired relocation of p100−/− MZ B cells in response to LPS.
Increased adhesion of p100−/− MZ B cells to VCAM-1. (A) Adhesion of MZ B cells to plate-bound recombinant Fc-VCAM-1 was measured as described in “Cell culture, detection of apoptotic cells, B-cell migration, and adhesion asays.” (B) Increased mRNA expression of cell-adhesion molecules in spleens from p100−/− mice. Total RNA from wild-type and p100−/− spleens was analyzed for VCAM-1, ICAM-1, and MAdCAM-1 expression by QRT-PCR. Relative mRNA expression corrected for β-actin levels is shown. Error bars indicate SD from 3 to 4 experiments. Significant differences are indicated (Student t test, *P < .05; **P < .01). Immunohistochemical staining of spleen sections from wild-type and p100−/− mice for (C) VCAM-1 (DAB brown), (D) MZMs (MARCO+; DAB brown), and (E) MAdCAM-1 (MECA-367+; Vector Blue). Sections in panels C and D were counterstained with hematoxylin. (F) Immunohistochemical staining of spleen sections (MARCO+, DAB brown; MECA-367+, Vector Blue) from lethally irradiated C57BL/6 mice that were reconstituted with wild-type (left panel) or p100−/− bone marrow (right panel). Images were acquired as in Figure 2E, with 20×/0.70 objective (C), 10×/0.40 objective (D,E), and 40×/0.75 objective (F).
Increased adhesion of p100−/− MZ B cells to VCAM-1. (A) Adhesion of MZ B cells to plate-bound recombinant Fc-VCAM-1 was measured as described in “Cell culture, detection of apoptotic cells, B-cell migration, and adhesion asays.” (B) Increased mRNA expression of cell-adhesion molecules in spleens from p100−/− mice. Total RNA from wild-type and p100−/− spleens was analyzed for VCAM-1, ICAM-1, and MAdCAM-1 expression by QRT-PCR. Relative mRNA expression corrected for β-actin levels is shown. Error bars indicate SD from 3 to 4 experiments. Significant differences are indicated (Student t test, *P < .05; **P < .01). Immunohistochemical staining of spleen sections from wild-type and p100−/− mice for (C) VCAM-1 (DAB brown), (D) MZMs (MARCO+; DAB brown), and (E) MAdCAM-1 (MECA-367+; Vector Blue). Sections in panels C and D were counterstained with hematoxylin. (F) Immunohistochemical staining of spleen sections (MARCO+, DAB brown; MECA-367+, Vector Blue) from lethally irradiated C57BL/6 mice that were reconstituted with wild-type (left panel) or p100−/− bone marrow (right panel). Images were acquired as in Figure 2E, with 20×/0.70 objective (C), 10×/0.40 objective (D,E), and 40×/0.75 objective (F).
Constitutive activation of the alternative NF-κB pathway in p100−/− mice results in a disorganized splenic MZ
The MZ is also characterized by the presence of two distinct macrophage populations, MZMs and MMMs. These specialized macrophages can be discriminated by staining for MARCO (macrophage receptor with collagenous structure) and the MOMA-1 mAb, respectively.32,33 Both macrophage populations were present in p100−/− spleens. The outer ring of MARCO+ MZMs appeared broadened in p100−/− compared with wild-type spleens (Figure 6D). Similarly, the inner ring of MOMA-1+ MMMs appeared less compact and more widely distributed in the absence of the p100 inhibitor (Figure 5A; data not shown).
The increased MAdCAM-1 mRNA levels in p100−/− spleens prompted us to stain sections for MECA-367+ stromal marginal sinus-lining cells.12 In wild-type spleens, the MECA-367 mAb clearly delineated the marginal sinus, separating red and white pulp. In striking contrast, staining of MAdCAM-1 in p100−/− spleen sections was strongly increased but dislocated to the red pulp, suggesting the lack of a continuous marginal sinus (Figure 6E). To distinguish between stromal and hematopoietic effects, we analyzed spleens from wt → B6 and p100−/− → B6 chimeras. As shown in Figure 6F, transfer of p100−/− bone marrow resulted in normal expression levels and distribution of MAdCAM-1 in the white pulp adjacent to MARCO+ MZMs, which appeared more loosely organized than in wt → B6 controls. These data indicate that constitutive activation of the alternative NF-κB pathway in stromal cells, probably via p52-RelB heterodimers,21,22 results in increased MAdCAM-1 mRNA expression and formation of ectopic MAdCAM-1+ structures in the splenic red pulp rather than a marginal sinus. The broadened distribution of MARCO+ MZMs in p100−/− spleens, however, was likely due to hematopoietic defects.
Lack of p100 induces HEV-like structures in the splenic red pulp
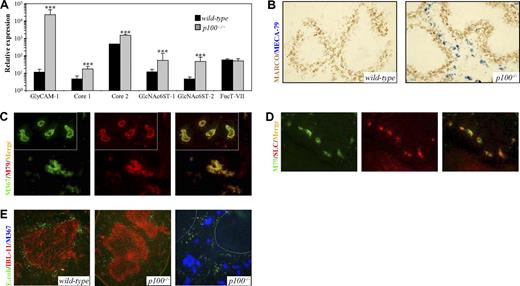
In addition to marginal sinus-lining cells in the spleen, MAdCAM-1 is also expressed in HEVs from PPs as well as from mesenteric and early peripheral LNs.13,34 Adult peripheral LN HEVs express highly glycosylated and sulfated forms of sialomucins, such as GlyCAM-1. Generation of the PNAd/MECA-79 epitope on GlyCAM-1 requires the action of the core 1 extension enzyme β3GlcNAcT-3 and the core 2 branching enzyme Core2GlcNAcT-I.14 Both core 1 extension and core 2 branch are capped by 6-sulfo sialyl Lewis x (sLex). These modifications are added by sulfotransferases GlcNAc6ST-1 and, in particular, GlcNAc6ST-2. Expression of GlcNAc6ST-2, also known as HEC-GlcNAc6ST or LSST, is highly restricted to LN and tonsilar HEVs.14 GlcNAc6ST-1 and GlcNAc6ST-2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in HEVs.35,36 The α(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment, and FucT-VII is the principle enzyme involved.37 Expression analysis of genes that are involved in PNAd epitope generation revealed a dramatic induction (2000-fold) of GlyCAM-1 mRNA levels in p100−/− compared with wild-type spleens. Expression of β3GlcNAcT-3 (core 1) and Core2GlcNAcT-I was moderately increased (3- to 4-fold). Moreover, GlcNAc6ST-1 and GlcNAc6ST-2 mRNA levels were up-regulated 5- and 10-fold, respectively. Both FucT-IV and FucT-VII were expressed at significant levels in the spleen, and no difference between wild-type and p100−/− mice was observed (Figure 7A; data not shown).
Lack of p100 results in the induction of ectopic HEV-like structures in the splenic red pulp. (A) QRT-PCR analysis of GlyCAM-1, β3GlcNAcT-3 (core 1), Core2GlcNAcT-I (core 2), GlcNAc6ST-1, GlcNAc6ST-2, and FucT-VII in spleens from wild-type and p100−/− mice. Note the log scale of relative mRNA expression. Error bars are SD. Significant differences are indicated (Student t test, ***P < .002). (B) Immunohistochemical staining of wild-type and p100−/− spleen sections for MZMs (anti-MARCO; DAB brown) and MECA-79 (Vector Blue). (C) 2-color immunofluorescence of p100−/− spleen sections stained with MECA-367 (M367; FITC green) and MECA-79 mAbs (M79; Texas red). Most MECA-367+ structures exhibited coexpression of PNAd (merge; yellow fluorescence). Insets, mesenteric LN HEVs from p100−/− mice. (D) 2-color immunofluorescence of p100−/− spleen sections stained with MECA-79 (M79; FITC green) and anti-CCL21 (SLC; Texas red). Most MECA-79+ structures exhibited coexpression of CCL21 (merge; yellow fluorescence). (E) Spleen sections from p100−/− mice (middle panel) and control littermates (left panel) that were intravenously injected with FITC-labeled E. coli were stained with the IBL-11 mAb (red) to indicate the white pulp. The right panel shows FITC-E. coli in p100−/− spleen sections stained with MECA-367 (Vector Blue) to indicate ectopic HEV-like structures. Dotted lines mark the boundary of the white pulp. Images were acquired as in Figure 2E, with 20×/0.70 objective (B), 40×/0.75 objective (C,D); 20×/0.70 objective (panel E left and middle), 40×/0.75 objective (panel E right).
Lack of p100 results in the induction of ectopic HEV-like structures in the splenic red pulp. (A) QRT-PCR analysis of GlyCAM-1, β3GlcNAcT-3 (core 1), Core2GlcNAcT-I (core 2), GlcNAc6ST-1, GlcNAc6ST-2, and FucT-VII in spleens from wild-type and p100−/− mice. Note the log scale of relative mRNA expression. Error bars are SD. Significant differences are indicated (Student t test, ***P < .002). (B) Immunohistochemical staining of wild-type and p100−/− spleen sections for MZMs (anti-MARCO; DAB brown) and MECA-79 (Vector Blue). (C) 2-color immunofluorescence of p100−/− spleen sections stained with MECA-367 (M367; FITC green) and MECA-79 mAbs (M79; Texas red). Most MECA-367+ structures exhibited coexpression of PNAd (merge; yellow fluorescence). Insets, mesenteric LN HEVs from p100−/− mice. (D) 2-color immunofluorescence of p100−/− spleen sections stained with MECA-79 (M79; FITC green) and anti-CCL21 (SLC; Texas red). Most MECA-79+ structures exhibited coexpression of CCL21 (merge; yellow fluorescence). (E) Spleen sections from p100−/− mice (middle panel) and control littermates (left panel) that were intravenously injected with FITC-labeled E. coli were stained with the IBL-11 mAb (red) to indicate the white pulp. The right panel shows FITC-E. coli in p100−/− spleen sections stained with MECA-367 (Vector Blue) to indicate ectopic HEV-like structures. Dotted lines mark the boundary of the white pulp. Images were acquired as in Figure 2E, with 20×/0.70 objective (B), 40×/0.75 objective (C,D); 20×/0.70 objective (panel E left and middle), 40×/0.75 objective (panel E right).
To investigate whether the increased expression of the sialomucin-modifying enzymes results in the generation of the PNAd/MECA-79 epitope, we stained spleen sections with MECA-79 together with anti-MARCO to indicate the MZ. As shown in Figure 7B, no MECA-79 staining could be detected in wild-type spleens, whereas p100−/− spleens showed MECA-79 staining in the red pulp. When p100−/− spleen sections were analyzed with both MECA-367 and MECA-79, a remarkable overlap of staining was observed, although the splenic HEV-like structures appeared less well organized compared with HEVs from mesenteric LNs (Figure 7C). Also, strong expression of CCL21, which is produced by most HEVs, was detected in the p100−/− splenic red pulp, coinciding with MECA-79 and MECA-367 staining (Figure 7D).
To address whether the marginal sinus and the ectopic HEV-like structures are functional, we intravenously injected FITC-labeled E. coli bacteria into p100−/− mice and control littermates. In control animals, the bacteria localized predominantly in the MZ adjacent to the reticular fibroblast network of the splenic white pulp.38 In contrast, localization of bacteria in p100−/− spleens was more diffuse, and distribution was even between red pulp and MZ (Figure 7E). Moreover, the bacteria in the red pulp of p100−/− spleens were not associated with the ectopic HEV-like structures. We also performed a modified Stamper-Woodruff in vitro adhesion assay39 to examine whether exogenous lymphocytes adhere to the HEV-like structures in p100−/− spleens. While only a very few lymphocytes adhered to wild-type spleens, increased numbers were bound to these structures on p100−/− spleen sections (Figure S5). However, adherence was clearly reduced compared with HEVs from wild-type or p100−/− LNs (Figure S5 insets). Collectively, these data indicate that constitutive alternative NF-κB signaling due to the lack of the p100 inhibitor, through the induction of the PNAd-generating pathway, results in the ectopic induction of functionally impaired HEV-like structures in the splenic red pulp at the expense of a normal marginal sinus.
Discussion
We have studied the consequences of signal-independent, constitutive activation of the alternative NF-κB pathway on the splenic MZ using p100-deficient knock-in mice. We demonstrate that proper control of p100 processing and activation of p52-RelB is crucial for normal development of MZ microarchitecture and its constituents, such as MZ B cells, specialized macrophages, and marginal sinus-lining cells.
Contrary to nfkb2−/− mice, which lack both p100 and p52, MZ B cells strongly accumulated in the splenic MZ of p100−/− mice. It is important to note that p105−/− mice, which lack the inhibitory p105 precursor but still express p50,40 did not have increased numbers of MZ B cells (data not shown), further supporting the specific role of the p100 inhibitor. The expansion of p100−/− MZ B cells was, at least in part, due to cell-intrinsic mechanisms as suggested by competitive bone marrow chimeras. The origin of MZ B cells is not well understood. It is believed that precursors within the T2 B-cell compartment (T2-MZPs) further develop into mature MZ B cells.5 The lack of p100 promoted the generation of T2-MZP and MZ B cells without significantly affecting differentiation of T2-FPs into FO B cells. The expansion of p100−/− MZ B cells is unlikely to be due to increased proliferation, since p100−/− and wild-type MZ B cells proliferated similarly upon stimulation with LPS, anti-CD40, or anti-IgM (data not shown). We observed decreased apoptosis of unstimulated and LPS-treated p100−/− MZ B cells compared with wild-type controls, indicating that increased MZ B-cell survival may contribute to their accumulation in p100−/− spleens.
BAFF is an essential B-cell survival factor41 that activates both classical and alternative NF-κB pathways through one of its receptors, BAFF-R.42 BAFF overexpression results in increased survival and accumulation of transitional T2 and MZ B cells.43 Whereas the alternative, NF-κB2–dependent pathway enhances long-term B-cell survival, the classical, NF-κB1–dependent pathway promotes immunoglobulin class switching and generation of pathogenic antibodies.44 However, it was also shown that activation of classical NF-κB by constitutively active IKKβ renders B-cell survival independent of BAFF/BAFF-R interactions.45 Therefore, constitutive activation of the classical pathway can override the requirement for the alternative pathway for B-cell survival. Protection from spontaneous apoptosis of cultured splenic B cells was recently also shown for TRAF3−/− mice. Importantly, TRAF3, which interacts with BAFF-R, CD40, and LTβR, functions as a negative regulator of the alternative pathway, since lack of TRAF3 results in degradation of p100 and production of p52 without stimulation.46 The consequences for MZ B cells were not addressed in this report. Thus, our data are in line with the model that lack of p100 in T2 and MZ B cells reflects constitutive activation of alternative NF-κB downstream of BAFF-R, resulting in decreased apoptosis and increased survival.
We also provide evidence that cell-intrinsic mechanisms as well as increased stromal expression of VCAM-1 outside the splenic white pulp contributed to the accumulation of MZ B cells in p100−/− spleens. The markedly increased expression of both VCAM-1 mRNA and protein in p100−/− spleens suggests that regulation of VCAM-1 does not only rely on classical NF-κB activity,30 but that the alternative NF-κB pathway is also involved. Among integrins examined on p100−/− MZ B cells, expression of β1 was slightly up-regulated compared with wild-type controls, correlating with moderately increased adhesion to VCAM-1. In addition to increased survival and retention in the MZ, p100−/− MZ B cells also showed impaired migration into follicles upon LPS injection. LPS-induced relocation of MZ B cells depends on CXCL13 signaling through its receptor CXCR5.47 A key function of S1P receptor signaling in MZ B cells is to overcome CXCL13-mediated recruitment into lymphoid follicles.8,9 MZ B cells from p100−/− spleens showed normal S1P-stimulated but impaired CXCL13-stimulated chemotaxis. These findings suggest that constitutive activation of the alternative NF-κB pathway interferes with CXCR5 signaling, whereas S1P receptor signaling is not affected. We have previously shown that RelB is essential for normal MZM development and proper localization of MMMs within the MZ.21,22 MARCO expressed on MZMs directly interacts with MZ B cells.11 MARCO+ MZMs were clearly present in p100−/− spleens distributed in a broadened ring within the MZ, raising the possibility that these macrophages provide additional signals that attract MZ B cells to the p100−/− MZ. Thus, different mechanisms contribute to the accumulation of MZ B cells in the spleens of p100−/− mice.
NF-κB signaling is essential for MZ B-cell development. Both NF-κB1/p50 and RelB are required in a cell-autonomous manner for the generation of MZ B cells.21,48 Loss of either RelA or c-Rel leads to a less severe reduction in MZ B cells.48 Here, we show that p52-deficient nfkb2−/− mice also have significantly reduced MZ B-cell numbers despite the combined lack of p100. These findings are in line with a recent report showing that both p50/NF-κB1 and p52/NF-κB2 are required to maintain the increased numbers of MZ B cells in BAFF-transgenic mice.44 RelB DNA-binding activity is clearly increased in several tissues from p100−/− mice.23 Deletion of one relB allele in p100-deficient mice (p100−/−relB−/+) restored RelB DNA binding to wild-type levels (data not shown) and largely reduced the accumulation of MZ B cells in the spleen. Thus, the level of RelB activity appears to be crucial for the regulation of MZ B-cell numbers. Moreover, loss of p50 in p100-deficient mice (p100−/−nfkb1−/−) resulted only in a minor reduction of MZ B-cell numbers compared with p100−/− spleens (data not shown). Together, these results indicate that p52-RelB complexes are largely responsible for the expansion of p100−/− MZ B cells.
We found strongly up-regulated MAdCAM-1 mRNA levels in p100−/− spleens, but we did not detect any MAdCAM-1+ marginal sinus-lining cells using the MECA-367 mAb. However, MAdCAM-1+ structures were detected in the splenic red pulp, and most of them were also positive for PNAd as shown by MECA-79 staining. Moreover, a dramatic induction of GlyCAM-1 mRNA levels along with increased expression of enzymes that participate in the synthesis of the PNAd epitope on HEVs was observed. Also, CCL21, the only chemokine that is expressed in endothelial cells of HEVs, was clearly detected in PNAd+ structures in p100−/− splenic red pulp. The reason for the loss of a MAdCAM-1+ marginal sinus in p100−/− spleens is unclear. At birth, MAdCAM-1+ cells are dispersed throughout the spleen and formation of a ring-like marginal sinus is delayed, but not absent, in MARCO-deficient mice.49 Thus, additional signals distinct from MARCO are likely to be involved in migration of MAdCAM-1+ cells to the marginal sinus. These signals may be reduced or absent in p100−/− spleens, trapping MAdCAM-1+ cells in the red pulp. Together with the increased expression of genes that are involved in the generation of the PNAd epitope, this may result in the induction of ectopic HEV-like structures in the p100−/− splenic red pulp. These structures, however, are unlikely to function as lymphocyte entry sites, since they appeared disorganized and showed only weak adhesion to lymphocytes compared with LN HEVs. Also, localization of intravenously injected bacteria in the MZ was reduced in p100−/− mice and did not correlate with the HEV-like structures in the red pulp. Together, these findings suggest impaired trafficking through the marginal sinus and offer a possible explanation for the markedly reduced spleen size and cellularity in p100−/− mice.23
During development of HEVs, all LNs express MAdCAM-1, but soon after birth, peripheral LN HEVs switch to PNAd, whereas mesenteric LNs continue to express MAdCAM-1 in addition to PNAd.34 The situation in the p100−/− spleen shows some similarities to LNs, suggesting the ectopic activation of a program that is normally restricted to LNs. In the current model of LN development, LTβR signaling in endothelial cells of HEVs engages the alternative NF-κB pathway by activating IKKα, followed by the subsequent transcription of HEV-specific genes, such as GlyCAM-1, GlcNAc6ST-2, and CCL21.16,50 This model has recently been supported by experiments, in which LT signaling was interrupted in adult mice. Expression of MAdCAM-1, GlyCAM-1, and key enzymes required for PNAd assembly is down-regulated in LNs upon LTβR-Ig injection.51 MAdCAM-1 expression can be induced by tumor necrosis factor (TNF) and regulatory NF-κB sites have been identified in its promoter,52 indicating that p52-RelB may directly regulate MAdCAM-1 expression. Although a direct regulation of the genes encoding GlcNAc6ST-2 and GlyCAM-1 by NF-κB has not yet been demonstrated, GlcNAc6ST-2 and GlyCAM-1 mRNA levels are significantly reduced in IKKAA and nfkb2−/− mice.16,53 Thus, it is likely that the cell-adhesion molecules MAdCAM-1 and GlyCAM-1 as well as enzymes of the PNAd-generating pathway are targets of p52-RelB regulation.
This study shows that constitutive activation of the alternative NF-κB pathway due to the lack of the p100 inhibitor favors the generation and retention of MZ B cells. Through the induction of key sialomucins and enzymes of the PNAd-generating pathway, it also results in the ectopic formation of HEV-like structures in the splenic red pulp and impaired function of the marginal sinus. Thus, tightly controlled NF-κB2/p100 processing, and consequently RelB activity, is essential for normal MZ B-cell numbers and proper organization of the splenic MZ.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge Simone Tänzer and Iwona Powolny for excellent technical assistance. We thank Rolf Gräbner for comments and Péter Balogh for the IBL-11 mAb and many helpful suggestions. We are indebted to Sabine Matz and all the staff in the animal facility of the Leibniz-Institute for Age Research—Fritz-Lipmann-Institute.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (grant We2224/4) (F.G. and F.W.).
Authorship
Contribution: F.G. designed and performed research, collected, analyzed, and interpreted data, and drafted the manuscript; D.W. designed and performed research, collected, analyzed, and interpreted data; E.M. performed research and collected data; and F.W. designed research, analyzed and interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Falk Weih, Leibniz-Institute for Age Research—Fritz-Lipmann-Institute, Research Group Immunology, Beutenbergstrasse 11, 07745 Jena, Germany; e-mail:fweih@fli-leibniz.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal