To examine whether donor-derived cells could exist in nonhematopoietic tissues of recipients after allogeneic hematopoietic stem-cell transplantation, we examined the patterns of the short tandem repeat (STR) of DNA extracted from fingernail clippings of recipients so that the contamination of blood cells was excluded. All 21 patients reached donor-derived hematopoiesis after transplantation and 20 of them were in remission of the primary diseases at the time of sampling. Compared with the STRs of donor cells, among 9 of 21 patients, DNA extracted from fingernail samples showed coexistence of the donor pattern of the STRs, sharing from 8.9% to 72.9% of total STR areas. Time from transplantation to sampling was from 305 to 2399 days among positive cases. These results demonstrate for the first time the existence of stable contribution of donor cells in fingernails among recipients of allogeneic hematopoietic stem cells.
Introduction
Among recipients of allogeneic hematopoietic stem cells (allo-HSCs), donor-derived cells in nonhematopoietic tissues have been observed by several groups.1,,–4 For example, in biopsy specimens such as skin, gastrointestinal mucosa, or liver that were taken mostly for the diagnosis of graft-versus-host disease (GVHD), cells carrying the Y chromosome were shown in nonblood cells (eg, epithelium of gastrointestinal tract) of female recipients who received transplants from male donors. These observations and reports of other tissues demonstrated that the cells existed in bone marrow or mobilized peripheral blood that could differentiate into (or contribute to) cells in organs or tissues other than those of hematopoietic lineage (eg, gastrointestinal mucosa, skeletal muscle, buccal mucosa, liver, and skin, etc).1,,,,,–7 On the other hand, there were also reports against the contribution, at least major contribution, of donor cells in nonhematopoietic cells in human and animal models.8,–10 One of the reasons for the discrepancy of these reports seemed to originate from methodological limitations.8,11 For example, in histologic analysis using a fluorescent DNA probe technique such as fluorescence in situ hybridization (FISH), coexistence of blood cells in the tissue samples made it difficult to distinguish true donor-derived cells from recipient cells that 3-dimensionally overlapped the donor-derived blood cells. If samples are taken from a biopsy specimen for the diagnosis of GVHD, they are small in general, which makes it difficult to perform a full analysis of chimerism. To overcome these problems and to examine the existence of donor-derived cells in nonhematopoietic tissue, we investigated the contribution of donor-derived cells in fingernails, the tissue without blood cells, among recipients of allo-HSCs. This is the first report to examine the donor-derived DNA in fingernails.
Patients, materials, and methods
The protocol of this study was approved by the Internal Review Board of Nagasaki University. Twenty-one recipients of allo-HSCs participated in this study and were treated and followed at the Department of Hematology, Nagasaki University Hospital. To examine the existence of donor-derived cells in fingernails, the short tandem repeat (STR) of genomic DNA extracted from fingernail clippers was compared with STRs of donor and recipient cells. After obtaining written informed consent in accordance with the Declaration of Helsinki, fingernail samples of 10 fingers (21 cases) and peripheral blood of participants (9 cases) were collected. A new nail cutter was used for every collection of fingernail samples in each patient to avoid contamination. All pieces of nail from each patient were subjected to extraction of total genomic DNA with proteinase K and phenol/chloroform. From nonseparated total peripheral blood, total genomic DNA was extracted and used to examine the chimerism of hematopoiesis at the time of fingernail sampling in 9 cases. The STR of 10 regions was determined using AmpFlSTR SGM Plus (Applied Biosystems, Foster City, CA). Polymerase chain reaction (PCR) was performed using GeneAmp PCR system 9600 (Applied Biosystems), and the difference of STRs was detected using an ABI PRISM 310 Genetic Analyzer and Genotyper software version 2.5 (Applied Biosystems). These procedures followed the instructions of the manufacturer. The contribution of donor-derived cells was described as a percentage of an average of donor peak areas divided by total peak areas of every different STR site (except for sex chromosome). The minimum sensitivity of this method was 5%. The Mann-Whitney U test (age and time from HSC transplantation [HSCT] to sampling) and the chi-square test (other factors) were used to test the statistical relationship with the existence of chimerism in nail.
Results and discussion
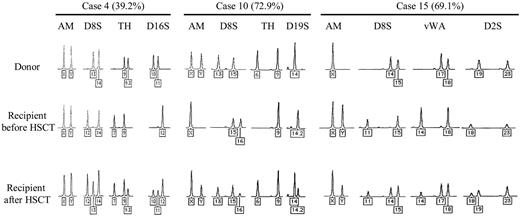
Table 1 shows the characteristics of donors and recipients, transplantation-related factors, and the percentages of donor-derived DNA in fingernails. All cases demonstrated complete donor chimerism in bone marrow cells within 100 days from transplantation, and there was no unexplained cytopenia in 17 cases. Among 4 cases (cases 2, 3, 11, and 21) that experienced relapse of primary diseases, 3 of them were in complete donor-derived hematopoiesis at the time of sampling. No case showed abnormal appearance of fingernails, including bleeding, when collected. In case 2, which later died of primary disease, fingernails were collected during partial remission. The other 20 patients were alive and in complete remission when this manuscript was written. In 9 of 21 cases, donor-derived STR peaks were detected in DNA samples of fingernails that shared from 8.9% to 72.9% of total peak areas. Representative STR peaks are shown in Figure 1. There was no statistically significant relationship between the presence of donor-derived STR peaks with mismatch of sex, disparity of blood type, or all factors listed in Table 1. However, because of the small number of cases in this study, we could not deny any relationship among the factors above. The only case that underwent transplantation after reduced-intensity conditioning showed no donor-derived STR in fingernails. So far there is 1 report that used PCR-based genotyping to show the positive contribution of donor-derived cells in nonhematopoietic tissue, buccal epithelial cells.4 However, the detection and the comparison of STRs of blood or bone marrow samples are established and widely accepted methods for the analysis of chimerism after allo-HSCT, and fingernails have also been used to extract DNA for this type of analysis.12,13 We did not observe any other peaks than those of recipients or donors in any STR test, demonstrating no contamination of other DNA samples. Taking advantage of the integration of HTLV-1 in T cells among adult T-cell leukemia/lymphoma (ATLL) patients, the existence of HTLV-1 in DNA of fingernails was examined by the genomic PCR of the pX region among 9 ATLL patients, resulting in no amplification in all cases (Figures S1, S2, available on the Blood website; see the Supplemental Materials link at the top of the online article). It suggested that no white blood cell (WBC)–derived DNA existed in DNA extracted from fingernails (data not shown). We believe there was little chance of false-positive results in our analysis.
Characteristics of hematopoietic stem-cell transplant recipients
| Case no. . | Diagnosis . | Age at HSCT, y . | Type of donor . | HLA allele compatibility . | Conditioning regimen for HSCT . | Source of HSCs . | aGVHD . | cGVHD . | Time from HSCT to sampling, d . | cGVHD at sampling . | Immunosuppressive therapy at sampling . | Percentage of donor area . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ALL | 26 | Unrelated | Match | CSI+CY+TBI | BM | II | Limited | 521 | Negative | None | 17.2 |
| 2 | AML | 53 | Related | Match | BU+CY | BM | None | Limited | 2529 | Positive | None | 0 |
| 3 | AML | 42 | Related | Match | BU+CY | BM | II | Limited | 569 | Negative | None | 0 |
| 4 | AML | 23 | Unrelated | Match | TBI+CY | BM | None | Limited | 1259 | Negative | None | 39.2 |
| 5 | AML | 45 | Related | Match | BU+CY | BM | I | Limited | 1657 | Negative | None | 23.8 |
| 6 | MDS | 18 | Related | Match | BU+CY | PB+BM | None | Extensive | 1811 | Positive | CsA | 0 |
| 7 | SAA | 23 | Related | Match | BU+CY | BM | I | Extensive | 2287 | Positive | FK | 15.1 |
| 8 | CML | 25 | Related | Match | BU+CY | PB | I | Extensive | 2252 | Positive | PSL | 0 |
| 9 | ALL | 55 | Unrelated | 2 locus mismatch | TBI+Flu+L-PAM | CB | I | Limited | 467 | Negative | None | 0 |
| 10 | ALL | 17 | Related | Match | TBI+CY | BM | I | Extensive | 999 | Positive | CsA+PSL | 72.9 |
| 11 | AML | 45 | Unrelated | 1 locus mismatch | TBI+CY | BM | None | Limited | 550 | Positive | None | 0 |
| 12 | AML | 33 | Unrelated | Match | CSI+CY+TBI | BM | II | Extensive | 1711 | Negative | None | 0 |
| 13 | AML | 26 | Related | Match | BU+CY | BM | None | Extensive | 305 | Positive | CsA+PSL+MMF | 8.9 |
| 14 | AML | 30 | Related | Match | BU+CY | BM | II | Extensive | 1580 | Positive | FK+PSL+MMF | 0 |
| 15 | ALL | 20 | Related | Match | BU+CY | BM | I | Limited | 1111 | Negative | None | 69.1 |
| 16 | ATL | 40 | Related | Match | TBI+CY | PB | None | Extensive | 1518 | Positive | CsA | 31.2 |
| 17 | AML | 49 | Unrelated | Match | TBI+CY | BM | None | Extensive | 426 | Positive | FK+PSL | 0 |
| 18 | ALL | 28 | Unrelated | 4 locus mismatch | CY+Flu+BU | CB | II | Extensive | 851 | Positive | None | 0 |
| 19 | AML | 17 | Related | Match | TBI+CY | PB | II | Extensive | 2107 | Positive | FK | 0 |
| 20 | AML | 42 | Related | Match | BU+CY | PB | I | Extensive | 1209 | Positive | FK+PSL | 0 |
| 21 | CML | 46 | Related | Match | BU+CY | BM | None | Limited | 2399 | Negative | None | 56.5 |
| Case no. . | Diagnosis . | Age at HSCT, y . | Type of donor . | HLA allele compatibility . | Conditioning regimen for HSCT . | Source of HSCs . | aGVHD . | cGVHD . | Time from HSCT to sampling, d . | cGVHD at sampling . | Immunosuppressive therapy at sampling . | Percentage of donor area . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ALL | 26 | Unrelated | Match | CSI+CY+TBI | BM | II | Limited | 521 | Negative | None | 17.2 |
| 2 | AML | 53 | Related | Match | BU+CY | BM | None | Limited | 2529 | Positive | None | 0 |
| 3 | AML | 42 | Related | Match | BU+CY | BM | II | Limited | 569 | Negative | None | 0 |
| 4 | AML | 23 | Unrelated | Match | TBI+CY | BM | None | Limited | 1259 | Negative | None | 39.2 |
| 5 | AML | 45 | Related | Match | BU+CY | BM | I | Limited | 1657 | Negative | None | 23.8 |
| 6 | MDS | 18 | Related | Match | BU+CY | PB+BM | None | Extensive | 1811 | Positive | CsA | 0 |
| 7 | SAA | 23 | Related | Match | BU+CY | BM | I | Extensive | 2287 | Positive | FK | 15.1 |
| 8 | CML | 25 | Related | Match | BU+CY | PB | I | Extensive | 2252 | Positive | PSL | 0 |
| 9 | ALL | 55 | Unrelated | 2 locus mismatch | TBI+Flu+L-PAM | CB | I | Limited | 467 | Negative | None | 0 |
| 10 | ALL | 17 | Related | Match | TBI+CY | BM | I | Extensive | 999 | Positive | CsA+PSL | 72.9 |
| 11 | AML | 45 | Unrelated | 1 locus mismatch | TBI+CY | BM | None | Limited | 550 | Positive | None | 0 |
| 12 | AML | 33 | Unrelated | Match | CSI+CY+TBI | BM | II | Extensive | 1711 | Negative | None | 0 |
| 13 | AML | 26 | Related | Match | BU+CY | BM | None | Extensive | 305 | Positive | CsA+PSL+MMF | 8.9 |
| 14 | AML | 30 | Related | Match | BU+CY | BM | II | Extensive | 1580 | Positive | FK+PSL+MMF | 0 |
| 15 | ALL | 20 | Related | Match | BU+CY | BM | I | Limited | 1111 | Negative | None | 69.1 |
| 16 | ATL | 40 | Related | Match | TBI+CY | PB | None | Extensive | 1518 | Positive | CsA | 31.2 |
| 17 | AML | 49 | Unrelated | Match | TBI+CY | BM | None | Extensive | 426 | Positive | FK+PSL | 0 |
| 18 | ALL | 28 | Unrelated | 4 locus mismatch | CY+Flu+BU | CB | II | Extensive | 851 | Positive | None | 0 |
| 19 | AML | 17 | Related | Match | TBI+CY | PB | II | Extensive | 2107 | Positive | FK | 0 |
| 20 | AML | 42 | Related | Match | BU+CY | PB | I | Extensive | 1209 | Positive | FK+PSL | 0 |
| 21 | CML | 46 | Related | Match | BU+CY | BM | None | Limited | 2399 | Negative | None | 56.5 |
ALL indicates acute lymphoblastic leukemia; CSI, craniospinal irradiation; CY, cyclophosphamide; TBI, total body irradiation; BM, bone marrow; AML, acute myelogenous leukemia; BU, busulfan; MDS, myelodysplastic syndrome; PB, peripheral blood; CsA, cyclosporine A; SAA, severe aplastic anemia; FK, tacrolimus; CML, chronic myelogenous leukemia; PSL, prednisolone; Flu, fludarabine; L-PAM, melphalan; CB, cord blood; MMF, mycophenolate mofetil; and ATL, adult T-cell leukemia.
Representative STR patterns of donor blood cells (top), those of recipients before HSCT (middle), and those of nails in recipients after HSCT (bottom) are shown (cases 4, 10, and 15). The percentages in parenthesis were calculated by dividing the donor-derived short tandem repeat (STR) areas by the total STR peak areas in nails of recipients. The numbers of the fragment repeats are indicated below each STR. AM indicates amelogenin gene; D8S, D8S1179; TH, TH01; D16S, D16S539; D19S, D19S433; vWA, von Willebrand factor intron A; and D2S, D2S1338.
Representative STR patterns of donor blood cells (top), those of recipients before HSCT (middle), and those of nails in recipients after HSCT (bottom) are shown (cases 4, 10, and 15). The percentages in parenthesis were calculated by dividing the donor-derived short tandem repeat (STR) areas by the total STR peak areas in nails of recipients. The numbers of the fragment repeats are indicated below each STR. AM indicates amelogenin gene; D8S, D8S1179; TH, TH01; D16S, D16S539; D19S, D19S433; vWA, von Willebrand factor intron A; and D2S, D2S1338.
The percentages of donor-derived STR areas were correlated to other reports,1,,–4 even a long time after transplantation, demonstrating the stable contribution of donor-derived cells in fingernails. Since nail is a tissue that regenerates continuously throughout life maintained by the stem cells of the nail matrix, the stable contribution of donor-derived DNA in nails suggested the existence of donor-derived cells in the stem-cell system of nails. Based on the clinical observation, when patients are treated with myeloablative conditioning regimen for transplantation, nail stem cells would be damaged to a certain extent so that transient growth retardation or arrest occurs. This harmful condition might facilitate the high contribution of donor-derived cells to nails.14 In our approach, there is no data available regarding how donor-derived cells resided in these patients: donor-derived nail stem cells existed or the fusion of certain donor cells occurred to nail stem cells of the recipients. We also have no data on the difference of the contribution of donor cells in each finger.
Recently, the absence of donor-derived cells was reported in the hair bulb of allo-HSC recipients (115 cases) where there was no contamination of blood cells either.9 In spite of the similar biologic features of hair and nail such as continuous regeneration or the same origin from the ectoderm, the contribution of donor-derived cells was clearly different between these 2 tissues after allo-HSCT. There is no clear explanation for the difference; however, biologic characteristics of stem cells and niche circumstances might contribute to these results. This report for the first time demonstrated the long-term, stable contribution of donor-derived cells in nails among recipients of allo-HSCs.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the Ministry of Health, Welfare, and Labor of Japan.
Authorship
Contribution: D.I., H.S., and R.Y. performed experiments with H.T., and Y.S. and J.T. collected samples. T.F., S.Y., and T.H. collected clinical data. Data were analyzed by D.I. and Y.M. Y.M. organized this study and wrote the manuscript with M.T.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yasushi Miyazaki, 1-12-4 Sakamoto, Nagasaki 852-8523, Japan; e-mail:y-miyaza@nagasaki-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal