MVs are membrane blebs shed from the surface of activated cells. Although they were originally considered to be inert cellular debris, it is now known that MVs interact with cells through specific receptor-ligand interactions.1 MVs also transfer receptors, proteins, and bioactive lipids to target cells.1 In this issue of Blood, Deregibus and colleagues extend the functional repertoire of MVs by showing that they are vehicles for mRNA transport and the exchange of genetic codes.
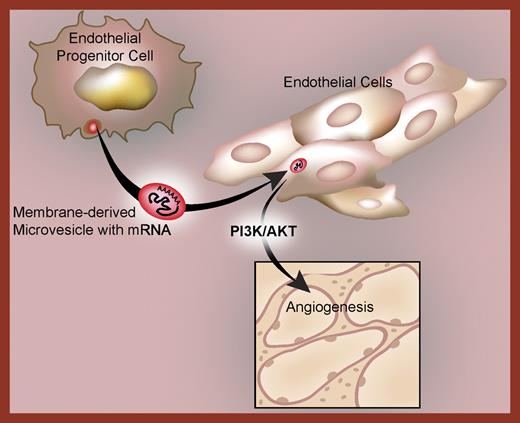
To demonstrate intercellular mRNA transfer, MVs were generated from endothelial progenitor cells and coincubated with endothelium. The MVs incorporate into target endothelial cells and induce angiogenesis. When the MVs are pretreated with RNase, however, they fail to exert any angiogenic effects even though they are internalized by the receiving cell. This indicates that subsets of the MV-derived mRNAs are translated into proteins that control neovas-cularization. As proof of this concept, the group found that green fluorescent protein (GFP) can be transduced in target endothelial cells by MVs loaded with GFP mRNA. Microarray profiling demonstrated that the MVs express distinct mRNAs including messages involved in PI3K/AKT signaling, and that inhibition of this signaling pathway blocked angiogenesis.
The studies raise a number of mechanistic questions and biologic issues that include the pathway(s) of MV entry into target cells, whether mRNAs activate translational control mechanisms during delivery, and whether there are recognition and sorting checkpoints for the transcripts. It is also not known if the mRNAs are packaged into MVs in a random or organized fashion. If the latter is true, it would suggest that the formation of MVs in the originating cell is a tightly regulated process that produces MVs with distinct transcriptome and proteome signatures. In vivo, platelets are the major source of MVs, and despite being anucleate, platelets contain thousands of mRNAs. Whether or not platelet-derived MVs contain and transfer mRNAs is not known—but similar to endothelial progenitors, platelet MVs are reported to induce proliferation, migration, and tube formation in target endothelial cells.2 This suggests that MVs generated from platelets, leukocytes, and other cells, including endothelial cells themselves, may have the same capacity. Previous work from other groups suggests that both the sources and targets of mRNA-containing MVs may be diverse. Ratajczak et al3 found that embryonic stem-cell–derived MVs reprogram hematopoietic progenitors by mRNA transfer. mRNA transfer has also been observed between tumor-derived MVs and monocytes.4
These observations3,4 also suggest that MVs may transfer infectious particles such as HIV and prions to other cells. They further raise the possibility that exogenous RNA and DNA modulate critical biologic and pathologic responses when they are incorporatedinto target cells. If so, then the mechanisms that cells use for dynamic exchanges, transfer events, and dialogs may be even more daedal and intricate than currently recognized, and if the specific mechanism of stem-cell MV transfer of mRNA to endothelial cells3 occurs in vivo, it may provide a new pathway to angiogenesis in human disease (see figure).
Microvesicles deliver a subset of mRNAs to target endothelial cells. Following their capture, the mRNAs are translated by the receiving endothelial cells into proteins that regulate angiogenesis through PI3K/AKT-dependent mechanisms.
Microvesicles deliver a subset of mRNAs to target endothelial cells. Following their capture, the mRNAs are translated by the receiving endothelial cells into proteins that regulate angiogenesis through PI3K/AKT-dependent mechanisms.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal