Abstract
High variability in drug response and a narrow therapeutic index complicate warfarin therapy initiation. No existing algorithm provides recommendations on refining the initial warfarin dose based on genetic variables, clinical data, and international normalized ratio (INR) values. Our goal was to develop such an algorithm. We studied 92 patients undergoing primary or revision total hip or knee replacement. From each patient we collected a blood sample, clinical variables, current medications, and preoperative and postoperative laboratory values. We genotyped for polymorphisms in the cytochrome P450 (CYP) 2C9 and vitamin K epoxide reductase (VKORC1) genes. Using stepwise regression, we developed a model for refining the warfarin dose after the third warfarin dose. The algorithm explained four fifths of the variability in therapeutic dose (R2adj of 79%). Significant (P > .05) predictors were INR value after 3 doses (47% reduction per 0.25-unit rise), first warfarin dose (+7% per 1 mg), CYP2C9*3 and CYP2C9*2 genotype (−38% and −17% per allele), estimated blood loss (interacting with INR3), smoking status (+20% in current smokers), and VKORC1 (−11% per copy of haplotype A). If validated, this model should provide a safer, more effective process for initiating warfarin therapy.
Introduction
Warfarin sodium is characterized by a narrow therapeutic range (eg, an international normalized ratio [INR]) of 2.0–3.0), a marked interindividual variation in dosing requirements, and an increased risk of adverse events when the dose is too high or low.1,2 To minimize the high incidence of such events,3-5 particularly during the first few weeks of initiating therapy,1,6 most guidelines recommend prescribing warfarin at or near the anticipated maintenance dose and then adjusting the dose by trial and error.1,7,8 While algorithms for predicting this maintenance dose a priori have improved,9-16 there remains little guidance on how this starting dose should be adjusted a posteriori based on the subsequent INR values. We hypothesized that use of genetic markers could help optimize these dose refinements.
Two common single nucleotide polymorphisms (SNPs) in the cytochrome P450 (CYP) 2C9 system are associated with impaired metabolism of warfarin,3-6,11,17 while SNPs in the gene for vitamin K epoxide reductase complex 1 (VKORC1) correlate with warfarin sensitivity and resistance.2,18-20 No prior study has examined the impact of these SNPs on warfarin-dose adjustments. Given the current knowledge about these markers, we hypothesize that for a given INR, a patient who is a slow metabolizer of warfarin may need a more cautious adjustment in their dose than a similar patient who is a normal metabolizer. Failure to tailor dose refinements during warfarin induction in poor metabolizers may have contributed to the 3-fold increased risk of (laboratory or clinical) adverse events among poor metabolizers in our initial prospective study of pharmacogenetic-based warfarin therapy.4
The purpose of this study was to develop a dose-refinement nomogram to guide clinicians in adjusting warfarin doses. This nomogram would be similar to prior algorithms,21,22 but will have 2 advantages: (1) it will allow for, but not require, a first dose that is tailored to clinical and/or genetic factors and (2) it will incorporate genetics and clinical factors that are independent predictors of how much the dose should be refined.1,11 If successful, the proposed warfarin nomogram would simplify and standardize warfarin initiation.
Patients, materials, and methods
The study was a retrospective analysis of 2 cohorts of orthopedic surgery patients who had participated in 2 prospective studies of pharmacogenetic-based warfarin therapy. The Human Research Protection Office at Washington University Medical Center approved these studies.
Patients
For patients in both cohorts, we offered participation if they were scheduled for primary or revision total knee or hip arthroplasty at Washington University Medical Center and if they were 18 years or older. We excluded patients who had previously taken warfarin or who had contraindications to warfarin treatment. To allow time for genotyping, we also excluded patients scheduled for surgery fewer than 7 days following referral to our anticoagulation service. As previously described,4 we recruited the first cohort (n = 46) between August 2003 and March 2004. We recruited the second cohort (n = 72) between February 2006 and July 2006. We excluded patients who stopped their warfarin therapy prior to becoming therapeutic (n = 26), leaving a final cohort of 92 participants. All patients provided written informed consent in accordance with the Declaration of Helsinki.
Data collection
We prospectively collected the following information: age, sex, race, ethnicity, height, weight, smoking status, current medications and history of hypertension, high blood pressure, cancer, gastrointestinal bleeding, and liver disease. We defined liver disease as cirrhosis, chronic hepatitis, or a 2-fold elevation in aspartate or alanine transaminase in the last 24 months. We estimated body surface area (BSA) from the classic formula.23 We used electronic records to obtain baseline INR values, preoperative and postoperative levels of creatinine, hematocrit, and platelets, estimated blood loss during surgery (EBL), and hemovac drainage following surgery. When EBL was recorded as “minimal,” we estimated a value of 50 mL.
Genotyping
We collected a 5-mL anticoagulated blood sample from each patient, centrifuged it, and isolated genomic DNA from buffy coat. We genotyped each patient for SNPs in the CYP2C9 and VKORC1 genes (listed with dbSNP reference SNP identifiers, as follows). Each sample was genotyped for the CYP2C9*2 (rs1799853) and CYP2C9*3 (rs1057910) alleles using the restriction fragment length polymorphism technique described previously.11 We tested each sample for VKORC1 SNPs 3673G>A (rs9923231; also designated −1639 G > A) and 6853G>C (rs8050894) using Pyrosequencing18 or 50 cycles of asymmetric polymerase chain reaction (PCR) using DNA isolated from 200 μL peripheral blood (0.5% of isolated DNA per genotype) with fluorescent minor groove binding probes (Nanogen, San Diego, CA; forward primer final concentration = 100 nM AA*AGA*CTCCTGTTA*GTTACCTC; reverse primer final concentration = 2 μM CCA*CTCCATGCAATCTTGGTGA; probe 1 final concentration = 400 nM Minor Groove Binder Eclipse Dark Quencher-CGCTCCGTGATGA-6-carboxyfluorescein; probe 2 final concentration = 400 nM Minor Groove Binder Eclipse Dark Quencher-ACGCTCGGTGA*T-2′,4′,1,4,-tetrachlorofluorescein, where each G* or A* is a modified proprietary base created by Nanogen that reduces G-G self association or improve stability of A-T bonding, respectively24 ) and melt curve analysis on a SmartCycler (Cepheid, Sunnyvale, CA). While blinded to clinical variables, we performed and interpreted genotyping in a clinical DNA diagnostic laboratory following CLIA guidelines.
Treatment
For both cohorts, the first warfarin dose was taken approximately 24 hours preoperatively and was tailored to clinical factors and some genetic factors. In the first cohort, we prescribed the first 2 warfarin doses based on CYP2C9 genotype and clinical factors, but rounded the first dose up to the nearest 5 mg, as previously described.4 In the second cohort, we prescribed the first dose (rounded to the nearest 0.5 or 1 mg) based on VKORC1 genotype and clinical factors using a pharmacogenetic algorithm that estimated initial dose (www.WarfarinDosing.org). In this cohort, we reduced the warfarin dose in patients with CYP2C9 variants beginning with the second dose. For inpatients, warfarin was taken at 5:00 pm and laboratory tests were drawn after midnight. After discharge, we recommended that warfarin be taken in the evening and commonly ordered morning INR draws by the home care nurses. Outpatients had INR tests drawn on Mondays and Thursdays and as clinically necessary. For most patients, we prescribed warfarin for 5 to 6 weeks if they were in the first cohort and for 4 to 5 weeks if in the second.
End point
The end point for this study was the therapeutic warfarin dose. We defined therapeutic dose as a dose that gave an INR in the target therapeutic range after 7 consecutive days or, where that never occurred (n = 7), after 6 consecutive days. In general, the target therapeutic range was 2 to 3 in cohort 1 and 1.7 to 2.7 in cohort 2.
Statistical analysis
To select candidate variables for the multiple regression model, we tested all variables for collinearity. Noncollinear variables and biologically plausible interaction terms were then tested for association with logarithmically transformed therapeutic warfarin dose using the stepwise regression procedure (PROC REG) in SAS (Version 9.0 for Windows; SAS Institute; Cary, NC). The natural logarithm (ln) was used for all logarithmic transforms.
We coded CYP2C9 SNPs as 0 (if wild type), 1 (heterozygous), and 2 (homozygous) to model additive allelic effects on warfarin dose. We coded 2 copies of the warfarin-sensitive VKORC1 haplotype A as 2, 1 copy as 1, and 2 copies of VKORC1 B haplotype as 0. Where haplotype-distinguishing SNP 3673G>A was missing (n = 4), we used the genotype at position 6853G>C on the basis of high pairwise linkage disequilibrium (D′ = .97) and our prior work with this SNP.18 Dummy variables were used to code for demographic factors (sex, race, ethnicity, and cohort), clinical variables (history of liver disease, hypertension, cancer, or gastrointestinal bleeding), specific habits (current smoking), and individual medications. We used continuous variables for the other factors (age, BSA, creatinine, preoperative INR, INR after 3 warfarin doses [INR3], preoperative warfarin dose, hemovac drainage, estimated blood loss [EBL], platelet count, and change in hematocrit). We logarithmically transformed EBL and INR3 because of their positively skewed distributions. Where EBL was missing from the electronic record (n = 19), we imputed it as a decreasing linear function of the ratio of postoperative (day 2) platelet count to preoperative platelet count. We also tested for INR3 interactions with CYP2C9*2, CYP2C9*3, and EBL.
While we set P > .3 thresholds for entry and exit into the stepwise regression, we considered statistically significant only those variables with P > .05 and a type II sum of squares contribution greater than .5. To prevent overfitting, the number of variables in the model, including the intercept, was constrained to be fewer than or equal to 10. This number included a variable representing the second warfarin dose, a variable representing an individual's target INR, and a variable representing VKORC1, all of which we forced into the regression model for clinical reasons. Because R2 can be inflated by the number of independent variables, we also present adjusted R2 (R2adj) for the model. In the final model, variance inflation factors were less than 3 for noninteracting terms, and less than 8 for interactions.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the paper as written.
Results
The mean age was 58 years, with a range of 21 to 83 years and median of 59 years (Table 1). The therapeutic daily warfarin dose ranged from 1.36 to 13.75 mg. The arithmetic mean dose was 5.4 mg/day and the geometric mean was 4.9 mg/day. The mean INR3 was 1.7 (SD = 0.5). EBL ranged from 50 mL to 4000 mL with an arithmetic mean of 564 mL and a geometric mean of 374 mL. Two percent and 22% of participants were CYP2C9*2 homozygotes and heterozygotes, respectively (Table 2), and 15% were CYP2C9*3 heterozygotes. Individuals with 1 or 2 copies of the VKORC1 haplotype A, represented 40% and 10% of the sample, respectively.
Demographic and clinical factors in the 92 participants reaching therapeutic dose
| Variable . | Value . |
|---|---|
| Age, mean (SD) | 58.2 (15.5) |
| BSA, m2, mean (SD) | 2 (0.3) |
| White, no. (%) | 79 (86) |
| African-American, no. (%) | 13 (14) |
| Female, no. (%) | 44 (48) |
| Therapeutic warfarin dose, geometric mean (SD) | 4.9 (2.5) |
| EBL, geometric mean (SD) | 376 (670) |
| INR0, geometric mean (SD) | 1.0 (0.1) |
| INR3, geometric mean (SD) | 1.6 (0.5) |
| Target INR, mean (SD) | 2.3 (0.2) |
| Warfarin dose, day before surgery, mean (SD) | 6.5 (2.3) |
| Warfarin dose, day of surgery, mean (SD) | 4.6 (1.7) |
| History of liver disease, no. (%) | 2 (2) |
| Smokes, no. (%) | 17 (18) |
| Takes amiodarone, no. (%) | 1 (1) |
| Takes simvastatin, no. (%) | 10 (11) |
| Takes fluvastatin, no. (%) | 7 (8) |
| Variable . | Value . |
|---|---|
| Age, mean (SD) | 58.2 (15.5) |
| BSA, m2, mean (SD) | 2 (0.3) |
| White, no. (%) | 79 (86) |
| African-American, no. (%) | 13 (14) |
| Female, no. (%) | 44 (48) |
| Therapeutic warfarin dose, geometric mean (SD) | 4.9 (2.5) |
| EBL, geometric mean (SD) | 376 (670) |
| INR0, geometric mean (SD) | 1.0 (0.1) |
| INR3, geometric mean (SD) | 1.6 (0.5) |
| Target INR, mean (SD) | 2.3 (0.2) |
| Warfarin dose, day before surgery, mean (SD) | 6.5 (2.3) |
| Warfarin dose, day of surgery, mean (SD) | 4.6 (1.7) |
| History of liver disease, no. (%) | 2 (2) |
| Smokes, no. (%) | 17 (18) |
| Takes amiodarone, no. (%) | 1 (1) |
| Takes simvastatin, no. (%) | 10 (11) |
| Takes fluvastatin, no. (%) | 7 (8) |
BSA indicates body surface area; EBL, estimated blood loss; INR0, international normalized ratio at baseline; and INR3, international normalized ratio after 3 warfarin doses.
Genotype-based frequencies and therapeutic doses
| SNP alleles . | No. (%) . | Mean therapeutic dose (SD), mg/day . |
|---|---|---|
| CYP2C9, WT / WT | 58 (63) | 6.1 (2.5) |
| CYP2C9*2 / WT | 17 (18) | 5.3 (2.3) |
| CYP2C9*2 /CYP2C9*2 | 2 (2) | 4.3 (2.4) |
| CYP2C9*3 / WT | 11 (12) | 3.3 (1.0) |
| CYP2C9*2 / CYP2C9*3 | 4 (4) | 2.6 (0.8) |
| CYP2C9*3 / CYP2C9*3 | 0 | — |
| VKORC1 | ||
| VKORC1 B / B | 45 (49) | 6.6 (2.5) |
| VKORC1 A / B | 37 (40) | 4.5 (2.0) |
| VKORC1 A / A | 10 (11) | 3.8 (1.7) |
| SNP alleles . | No. (%) . | Mean therapeutic dose (SD), mg/day . |
|---|---|---|
| CYP2C9, WT / WT | 58 (63) | 6.1 (2.5) |
| CYP2C9*2 / WT | 17 (18) | 5.3 (2.3) |
| CYP2C9*2 /CYP2C9*2 | 2 (2) | 4.3 (2.4) |
| CYP2C9*3 / WT | 11 (12) | 3.3 (1.0) |
| CYP2C9*2 / CYP2C9*3 | 4 (4) | 2.6 (0.8) |
| CYP2C9*3 / CYP2C9*3 | 0 | — |
| VKORC1 | ||
| VKORC1 B / B | 45 (49) | 6.6 (2.5) |
| VKORC1 A / B | 37 (40) | 4.5 (2.0) |
| VKORC1 A / A | 10 (11) | 3.8 (1.7) |
SD indicates standard deviation; WT, wild type; CYP2C9, cytochrome P450 2C9; —, not available; and VKORC1, vitamin K epoxide reductase complex 1.
The final model consisted of 8 factors significantly associated with higher therapeutic warfarin dose: lower INR3, higher first dose, fewer CYP2C9*2 and CYP2C9*3 copies, fewer VKORC1 haplotype A copies, an increase in the value of the product of EBL and INR3, and current smoking status (Table 3). Creatinine clearance was not significant in the multivariate model.
Multivariate analysis: independent predictors of therapeutic warfarin dose at day 3
| Entry into model . | Variable . | % Change in therapeutic warfarin dose (95% CI)* . | R2 after entry, % . | P in final model . |
|---|---|---|---|---|
| — | Intercept | — | — | < .001 |
| 1 | INR3 | −46.5 (−33.3 to −57.1) | 34.4 | < .001 |
| 2 | 1st warfarin dose, per mg | +7.1 (+4.0 to +10.4) | 54.7 | < .001 |
| 3 | CYP2C9*3, per allele | −38.1(−29.3 to −45.7) | 69.8 | < .001 |
| 4 | 2nd warfarin dose, per mg | +3.9 (+0.0 to +8.0) | 73.6 | .051 |
| 5 | CYP2C9*2, per allele | −17.4 (−8.3 to −25.6) | 75.6 | .001 |
| 6 | EBL × INR3 | +44.9 (+16.6 to +80.2) | 78.1 | .001 |
| 7 | Smokes | +20.1 (+6.0 to +36.2) | 79.9 | .005 |
| 8 | VKORC1 haplotype A, per copy | −10.7 (−2.0 to −18.6) | 81.1 | .018 |
| 9 | Target INR | +14.6 (−7.9 to +42.5) | 81.5 | .218 |
| Entry into model . | Variable . | % Change in therapeutic warfarin dose (95% CI)* . | R2 after entry, % . | P in final model . |
|---|---|---|---|---|
| — | Intercept | — | — | < .001 |
| 1 | INR3 | −46.5 (−33.3 to −57.1) | 34.4 | < .001 |
| 2 | 1st warfarin dose, per mg | +7.1 (+4.0 to +10.4) | 54.7 | < .001 |
| 3 | CYP2C9*3, per allele | −38.1(−29.3 to −45.7) | 69.8 | < .001 |
| 4 | 2nd warfarin dose, per mg | +3.9 (+0.0 to +8.0) | 73.6 | .051 |
| 5 | CYP2C9*2, per allele | −17.4 (−8.3 to −25.6) | 75.6 | .001 |
| 6 | EBL × INR3 | +44.9 (+16.6 to +80.2) | 78.1 | .001 |
| 7 | Smokes | +20.1 (+6.0 to +36.2) | 79.9 | .005 |
| 8 | VKORC1 haplotype A, per copy | −10.7 (−2.0 to −18.6) | 81.1 | .018 |
| 9 | Target INR | +14.6 (−7.9 to +42.5) | 81.5 | .218 |
The optimal pharmacogenetics algorithm that estimated therapeutic dose (mg/day) 3 days after surgery was as follows: Exp[1.0138 − 2.5047 × ln(INR3) + 0.0690 × 1st warfarin dose + 0.0385 × 2nd warfarin dose + 0.2474 x ln(EBL) × ln(INR3) − 0.1912 × CYP2C9*2 − 0.4793 × CYP2C9*3 + 0.1835 × Smokes − 0.1132 × VKORC1+ 0.2724 × Target INR], where ln(EBL) × ln(INR3) is the interaction between logarithm (EBL) and logarithm (INR3), and VKORC1 is the number of A haplotypes.
— indicates not available.
Percent change (95% confidence interval) was calculated for each 0.25-point increase in log(INR3) and 1.5-point increase in the interaction of ln(EBL) and ln(INR3).
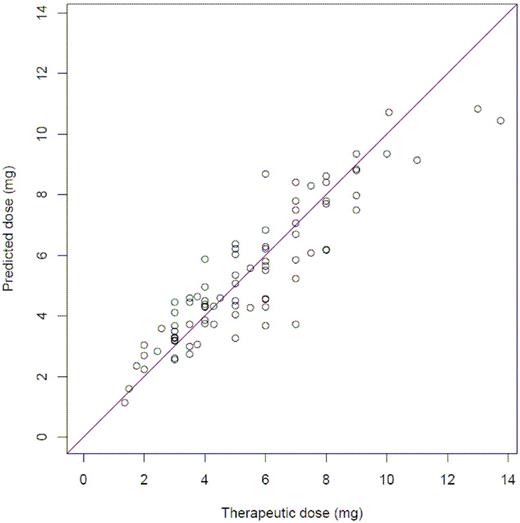
After including forced variables, the pharmacogenetic algorithm that best estimated therapeutic dose (mg/day) from data available on the third day after surgery was as follows: Exp[1.0138 − 2.5047 × ln(INR3) + 0.0690 × 1st warfarin dose + 0.0385 × 2nd warfarin dose + 0.2474 × ln(EBL) × ln(INR3) − 0.1912 × CYP2C9*2 − 0.4793 × CYP2C9*3 + 0.1835 × Smokes − 0.1132 × VKORC1 + 0.2724 × TargetINR], where Exp is the exponential function. In total, the pharmacogenetic model explained 81.5% of the variation in therapeutic warfarin dose (R2 = 81.5%; R2adj = 79.3%) (Table 3; Figure 1). History of liver disease was found nominally significant (P = .067) and was not included in the final model. Removing VKORC1, however, resulted in an alternative model capable of explaining 81.2% of the variation (R2 = 81.2%; R2adj = 79.0%) in which history of liver disease was statistically significant (P = .033): EXP[1.2091 − 0.1575 × CYP2C9*2− 0.4814 × CYP2C9*3 − 0.3610 × History of Liver Disease + 0.1939 × Smokes + 0.1084 × Target INR − 2.5682 × ln(INR3) + 0.0906 × 1st Warfarin Dose + 0.0405 × 2nd Warfarin dose + 0.2452 × ln(INR3) × ln(EBL)].
When the data were analyzed without the 19 patients for which EBL was imputed, the interaction of INR3 and blood loss remained significant (P = .012) with a similar impact on the dose estimation.
Discussion
The initiation of warfarin therapy carries a high risk of adverse events, particularly during the first weeks of therapy as the proper dose for each patient is determined.1,5,25-27 Current dosing algorithms attempt to minimize this risk by prescribing either a standard dose or an estimate of the maintenance dose. For either strategy, the actual maintenance dose is eventually found through a process of trial and error as the starting dose is adjusted according to the INR value—often starting on the third or fourth day of therapy.9,21,22,28 Our results demonstrate that the INR response is only one of several factors that should be used to estimate the therapeutic dose in orthopedic patients: the accuracy of dose refinements based solely on INR is inherently limited, heightening the chance of excessive or inadequate warfarin dose adjustments.
After INR response, the next variable to enter the stepwise regression was the initial warfarin dose, which explained 20.3% of the variability in therapeutic dose. This finding is interesting because 34 (37%) of the included patients were given initial doses that did not take VKORC1 genotype into account, while the remainder received genetically tailored doses. This observation suggests that our model should be appropriate for orthopedic patients, regardless of whether their genotype is known before the first warfarin dose. It is also important to note that the first dose was adjusted for age, BSA, and amiodarone in both cohorts. This adjustment helps explain the statistical significance of the first dose as well as the absence of these variables elsewhere in our model, given their known correlation with therapeutic dose.11,29-32
Consistent with previous studies,3-6,11-17 we also found that the CYP2C9*2 and *3 alleles were significant predictors of therapeutic dose. The therapeutic dose was 17.4% lower per *2 allele and 38.1% lower per *3 allele (Table 3). The significance of VKORC1 is also consistent with recent studies, which suggest an increased sensitivity to warfarin per copy of haplotype A.2,12-16,19,20 However, an alternative model that did not include VKORC1 was nearly as predictive as the full model, because much of the predictive power of VKORC1 genotype was captured in INR3. This raises the interesting question of whether genotyping for VKORC1 status is a necessary and cost-effective step when initiating warfarin therapy. We did not analyze any of the additional CYP2C9 alleles that may be associated with warfarin metabolism. However, a recent review found that of 30 genes possibly related to warfarin action and metabolism, CYP2C9 and VKORC1 are the ones that are clearly important.16 Thus, the inclusion of additional genes is unlikely to increase the predictive value of the model significantly.
Of interest, we found that the interaction between INR3 and EBL during surgery was significantly related to the eventual therapeutic dose, with the dose increasing 44.9% for every 1.5-point increase in the interaction term. This result must be interpreted carefully: rather than affecting the patient's long-term warfarin requirements, we profess that blood loss affects the value and interpretation of INR3. With blood loss during surgery, patients experience an acute loss of clotting factors that are not replenished via transfusions of packed red blood cells. This loss temporarily inflates the INR values commensurate with EBL. Indeed, though our current study focuses on orthopedic patients, this correlation may explain why postoperative patients are particularly sensitive to warfarin after valve-replacement surgery.33 This observation should be validated because we had to impute EBL from the change in platelets for 19 patients. Nevertheless, the association is unlikely to be spurious, as excluding those with imputed EBL values did not significantly alter the predictive weight of this variable.
Two clinically relevant variables were exposed as potential indicators of therapeutic dose. First, we found a significant impact on the therapeutic dose from smoking. The effect of smoking (+ 20.1%) was larger than expected, since hepatic enzyme induction from smoking results in only a 10% increase in warfarin clearance.29 Directionally, however, this is consistent with other studies that have shown a significant relationship between smoking status and warfarin dose.15 While nominally significant, the effect of a history of liver disease on therapeutic dose is clinically relevant and is consistent with previous studies linking liver disease to a decrease in warfarin requirements.34 This impact should be investigated further, as only 2 patients in our sample had liver disease.
In addition to requiring a cautious interpretation of the effects of EBL and liver disease variables, this study has other limitations. First, our study population consisted entirely of patients initiating warfarin for deep vein thrombosis prophylaxis following total hip or knee arthroplasty. The ability to generalize our model for other indications is unknown and should be studied in a broad population. In particular, the appropriate starting doses and the ability to safely initiate warfarin without genetic information need to be examined in other patient groups—including nonsurgical populations. Moreover, like any data-driven model, it might reflect peculiarities within our data rather than causal relationships between the variables and the therapeutic dose.35 In addition, our definition of therapeutic dose was ad hoc as definitions vary among studies.4,8,11,21,22 Our definition was appropriate for orthopedic patients who tend to use warfarin for a limited timeframe. A final minor limitation was that only 19 patients were taking a statin and only 1 was taking amiodarone, so we had inadequate statistical power to determine how these, and other drugs that interact with warfarin, affected warfarin dose.
Despite these limitations, this research has important implications about the timing and effectiveness of pharmacogenetics-based warfarin therapy in orthopedic patients, and potentially additional populations as well. Prior pharmacogenetic dosing algorithms explain 51% to 60% of the variability (R2) in warfarin dose,2,15,32,36,37 but implicitly expect the genotype to be available rapidly. Same-day genotyping is unlikely to be available in most settings, but may be unnecessary: even in poor metabolizers who carry one copy of the CYP2C9*2 or CYP2C9*3 alleles, S-warfarin plasma concentrations are subtherapeutic for at least 3 days.3 Moreover, while INR values rise more rapidly in patients with 2 variant CYP2C9 alleles, the rise may be slow enough to avoid excessive INR3 values (ie, INR3 < 3).38 However, additional study is needed to quantify how very poor warfarin metabolism affects the rate of INR rise. With frequent monitoring of INR values, a patient with extremely poor warfarin metabolism should be identifiable well before he/she reaches a dangerously supratherapeutic INR. Thus, provided the initial warfarin doses are not excessive and the INR is closely monitored, physicians may have a 3-day window between the time that they start warfarin therapy and the time when they need to know genotype. Although the efficacy of this approach should be demonstrated in a prospective cohort of nonorthopedic patients, the principle of a moderate initial dose followed by genetically tailored dose refinement should be broadly applicable. This knowledge, and the approach developed here, should allow for more effective and less expensive pharmacogenetics-based warfarin therapy.
The next steps are to make the new pharmacogenetics refinement algorithm accessible to clinicians and to validate it in a diverse population, including patients with nonsurgical indications for warfarin therapy. In pursuit of these goals, we have made an interactive and free version of this refinement algorithm available online at www.WarfarinDosing.org. Provided that the variables in Table 3 are available, the website uses the algorithm developed here. The website can estimate warfarin dose without INR3 and genotype, but the accuracy of these estimates is much less than that of the full model. The website also solicits information about key interacting drugs to provide relevant warnings about warfarin dose (and to quantify the relevance of these drugs in future research). In summary, www.WarfarinDosing.org allows clinicians to incorporate pharmacogenetics into their dosing plans—bearing in mind the experimental nature of this algorithm and its unproven applicability to nonorthopedic patients—and allows us to validate this pharmacogenetic approach in a diverse, anonymous population.
The results of this study contain a number of interesting possibilities for safe, effective, and efficient warfarin initiation. Incorporating clinical and genetic factors with the INR in determining warfarin dose refinements could substantially improve trial-and-error dose adjustments and reduce the risks of initiating warfarin therapy. Moreover, allowing physicians to initiate warfarin therapy while the genotype is being processed and then incorporating genetic information into a refinement after 3 doses would allow for offsite genotyping, thereby facilitating this approach in any setting. Ultimately, with further validation and refinement, this pharmacogenetic model should yield a streamlined approach to refining the dose and improving the safety and efficiency of warfarin initiation.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This research was funded by the NIH, R01 HL074724 and T35 HL007815.
National Institutes of Health
Authorship
Contribution: C.E. and B.F.G. designed research; E.A.M., P.E.M., C.E., G.G., J.C.C., R.L.B., R.S.J.B., D.V., S.G., A.T., and B.F.G. collected data; E.A.M., P.A.L., L.G., E.D., G.G., and B.F.G. analyzed and interpreted data; P.A.L. and E.D. performed statistical analysis; E.A.M., P.A.L., and B.F.G. drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Brian F. Gage, Campus Box 8005, Dept of Medicine, 660 South Euclid, St Louis, MO 63110; e-mail: bgage@im.wustl.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal