Abstract
Common lymphoid progenitors (CLPs) are lymphoid-lineage-committed progenitor cells. However, they maintain a latent myeloid differentiation potential that can be initiated by stimulation with interleukin-2 (IL-2) via ectopically expressed IL-2 receptors. Although CLPs express IL-7 receptors, which share the common γ chain with IL-2 receptors, IL-7 cannot initiate lineage conversion in CLPs. In this study, we demonstrate that the critical signals for initiating lineage conversion in CLPs are delivered via IL-2 receptor β (IL-2Rβ) intracellular domains. Fusion of the A region of the IL-2Rβ cytoplasmic tail to IL-7Rα enables IL-7 to initiate myeloid differentiation in CLPs. We found that Shc, which associates with the A region, mediates lineage conversion signals through the mitogen activated protein kinase (MAPK) pathway. Because mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) inhibitors completely blocked IL-2-mediated lineage conversion, MAPK activation, specifically via the MEK/ERK pathway, is critically involved in the initiation of this event. Furthermore, formation of granulocyte/macrophage (GM) colonies by hematopoietic stem cells, but not by common myeloid progenitors (CMPs), was severely reduced in the presence of MEK/ERK inhibitors. These results demonstrate that activation of MEK/ERK plays an important role in GM lineage commitment.
Introduction
Cytokines play important roles in regulating hematopoietic cell growth, survival and differentiation via their cognate cytokine receptors.1-3 Although the growth and survival signals provided by cytokine receptors have been studied extensively in various cytokine-dependent cell lines4-10 and transgenic animal models,11-13 a limited number of studies demonstrate the specific differentiation signals via cytokine receptors.14-17 Several recent reports suggest the involvement of cytokines in differentiation during hematopoiesis. For example, granulocyte colony-stimulating factor (G-CSF) receptor signaling has been demonstrated to be important for initiating granulocytic cell fate in granulocyte/macrophage (GM) bipotent progenitors.18 We also recently demonstrated the role of IL-7 receptor signaling in maintaining B-cell potential in pre-proB cells.19 However, it is not clear whether certain cytokines can initiate specific lineage commitment in hematopoietic stem cells (HSCs) or more mature progenitors, such as multipotent progenitors (MPPs).
During the course of maturation, HSCs gradually lose multipotency and undergo lineage commitment.20,21 Although both instructive and stochastic models have been proposed to explain the action of cytokines in the lineage decisions of HSCs and/or MPPs, recent studies with gene-modified mouse models have suggested that the role of lineage-specific cytokines (or colony-stimulating factors) is to support proliferation and survival of cells after lineage commitment in vivo.22-25 On the other hand, we and others have shown that ectopic interleukin-2 (IL-2) or granulocyte macrophage colony-stimulating factor (GM-CSF) stimulation can initiate latent myeloid differentiation from common lymphoid progenitors (CLPs).26,27 CLPs are progenitors that have committed to the lymphoid lineage and lack myeloid potential without gene manipulation.28 The lineage conversion observed in CLPs resulting from ectopic cytokine receptor signaling suggests that cytokines can have instructive actions under certain experimental settings. However, the mechanism by which IL-2 or GM-CSF receptor signaling directs lineage decision, as well as the physiologic relevance of this process, have not yet been clarified.
Under physiologic conditions, CLPs express IL-7 receptors and receive signals from IL-7 receptors continuously. It is noteworthy that although a majority of the signaling pathways activated via the IL-2 and IL-7 receptors are shared,29-31 only IL-2 stimulation can initiate GM differentiation in CLPs.26 This indicates that there must be differences between the signals provided by IL-2 and those from IL-7 receptors. IL-2 and IL-7 receptors both share the common γ (γc) chain as an indispensable component.32,33 In our experimental model with CLPs, ectopic human IL-2 receptor β (IL-2Rβ) and endogenous mouse γc form functional intermediate IL-2 receptors.34,35 Because functional IL-7 receptors expressed by CLPs are composed of IL-7Rα and γc, the different actions of IL-2 and IL-7 are most likely to be explained by differences between IL-2Rβ and IL-7Rα signaling. One of the candidate signaling molecules is the adaptor protein Shc. It has been demonstrated that Shc is phosphorylated following IL-2 but not IL-7 stimulation.36 In this study, we demonstrate that the Shc-mediated signaling pathway is sufficient to initiate latent myeloid differentiation in CLPs. We also demonstrate that Shc mediates lineage conversion by activating the mitogen-activated protein kinase (MAPK) pathway, specifically via the mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway. GM colony formation from HSCs but not from common myeloid progenitors (CMPs) was severely impaired in the presence of the MEK/ERK inhibitors. Therefore, the MEK/ERK pathway may play an important role in GM lineage commitment, not only in the context of ectopic IL-2-mediated lineage conversion in CLPs but also during physiologic hematopoiesis from HSCs.
Materials and methods
Mice
C57Bl/Ka-Thy1.1 mice at 6-10 weeks of age were used in this study. These mice were bred and maintained under specific pathogen-free environment at the Duke University Medical Center Animal Care Facility. All experimental procedures related to laboratory mice were preformed according to guidelines specified by the institution.
Construction of chimeric and mutant receptor subunits
The 7α/2β/2β, 7α/7α/7α-2βA, and 7α/7α/7α-2βA deletion chimeras and IL-2Rβ d325 deletion mutant were generated by polymerase chain reaction (PCR) using mouse IL-7Rα or human IL-2Rβ cDNAs as templates. To make fusion constructs, we used an overlapping PCR strategy that can be found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). The IL-2Rβd325, which was provided by Dr B. Nelson (Deeley Research Center, British Columbia Cancer Agency, Victoria, BC, Canada), is described.37 Tyrosine substitution mutants in the A region of 7α/7α/7α-2βA were generated by site-directed mutagenesis. The nucleotide sequences of synthesized oligonucleotides that are used in construction of receptor subunits and precise procedures can be found in Document S1. Constructs generated by PCR were confirmed by sequencing. All cDNAs were subcloned between the 5′ long terminal repeat and internal ribosomal entry site (IRES) in the murine stem cell virus (MSCV)-IRES-green fluorescent protein (GFP) vector. IL-2Rβ, IL-2RβA, and IL-7Rα on MSCV-IRES-GFP are described.19,26,38
Cell sorting and FACS analysis
Antibodies used in fluorescence-activated cell sorting (FACS) and analysis are as follows: fluorescein isothiocyanate (FITC)-anti-Thy-1.1 (HIS51), FITC-anti-CD34 (RAM34), phycoerythrin (PE)- or biotin-conjugated anti-IL-7Rα (A7R34), PE-anti-Flt3, PE-anti-FcγR (2.4G2), PE/Cy5- or allophycocyanin (APC)-conjugated anti-B220 (RA3–6B2), PE- or PE/Cy5-conjugated anti-Mac-1 (M1/70), PE/Cy5-conjugated anti-CD3 (145-2C11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), anti-Gr-1 (RB6–8C5), anti-Mac-1 (M1/70), anti-TER119, biotinylated anti-vascular cell adhesion molecule-1 (VCAM-1) (clone 429), and APC-anti-c-Kit (2B8). All of the above were purchased from either eBioscience (San Diego, CA) or BD Biosciences (San Jose, CA). Alexa Fluor (Invitrogen, Carlsbad, CA) 594-anti-Sca-1 was prepared in our laboratory with a standard procedure. Biotin-conjugated antibodies were visualized with PE or PE/Cy7-streptavidin (eBioscience).
CLPs, CMPs, VCAM-1−Flt3+ MPPs, and HSCs were doubly sorted from bone marrow as described previously.38-41 Sorting gates to purify these populations were set as shown in Figure S1. For cell surface phenotyping, cells were incubated with normal rat IgG (Sigma), followed by fluorescence- or biotin-conjugated antibodies on ice for 20 minutes. If necessary, cells were further incubated with fluorochrome-conjugated-streptavidin after washing with staining medium (Hanks' balanced salt solution with 2% fetal calf serum and 0.02% NaN3). FACS sorting and analysis were done on a FACSVantage with a DiVa option equipped with a 488 nm argon laser, a 599 nm dye laser, and a 408 nm krypton laser (BD Biosciences Flow Cytometry Systems), which is available in the FACS facility of Duke University Comprehensive Cancer Center. FACS analysis shown in Figure 3 was done on FACScan (BD Biosciences). Data were analyzed with the FlowJo software (Treestar, Ashland, OR). Dead cells were excluded from analyses and sorting as positively stained cells by propidium iodide.
Retroviral gene transfer and in vitro culture of CLPs
Retroviral production was done as described previously.26 For retroviral transduction, CLPs were cultured in X-VIVO 15 (Lonza Walkersville, Inc., Walkersville, MD) containing 10% FCS, stem cell factor (SCF) (50 ng/mL), Flt3L (30 ng/mL), IL-7 (10 ng/mL) together with viral stock at 37°C for 12-14 hours. For HSCs, X-VIVO 15 was supplemented with SCF (50 ng/mL), thrombopoietin (10 ng/mL), and IL-11 (10 ng/mL). After new viral supernatant was added, cells were spun at 2000 revolutions per minute (rpm), 25°C for 2 hours. Cells were then further cultured at 37°C for 24 hours for gene expression. When IL-2Rβ or its mutants were introduced into CLPs, human IL-2 (50 ng/mL) was further added to stimulate cells. One hundred GFP+ cells were purified by FACS sorting and cultured in either stromal cell cultures or methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada). Stromal cell cultures were set up in the presence of IL-3 (10 ng/mL) and GM-CSF (10 ng/mL) for GM readout or Flt3L (30 ng/mL) and IL-7 (10 ng/mL) for lymphoid readout as described previously.38 To detect GM colony-forming ability, MethoCult 3534 containing IL-3, IL-6, and stem cell factor with additional GM-CSF (10 ng/mL) was used. All data displayed in this article are representative of at least 3 independent experiments (except Figure 6E,G, which are from 2 independent experiments). The size of colonies from CLPs with various constructs on methylcellulose was not significantly different compared with the size from IL-2Rβ+ CLPs. Colony-forming cells from 7α/7α/7α-2βA+ CLPs contained immature myelocytic cells.
Establishment of CTLL-2 transfectants
An IL-2-dependent mouse T-cell line, CTLL-2, was cultured in the complete medium (RPMI 1640 medium with 10% FCS and 50 μM 2-mercaptoethanol) supplemented with 2 ng/mL hIL-2. CTLL-2 transfectants that stably express 7α/7α/7α, 7α/2β/2β, and 7α/7α/7α-2βA were established with retroviral systems, followed by purification of GFP+ cells by FACS. Expression of exogenously introduced genes was confirmed with staining of cell surface IL-7Rα (Figure 3A).
Proliferation assays
Spleen cells were cultured in the presence of 2.5 μg/mL concanavalin A (Con A) for 48 hours. CTLL-2 transfectants were maintained in complete medium supplemented with hIL-2. After washing 3 times with phosphate-buffered saline (PBS), 5 × 104 cells in complete medium were cultured in a well of 96-well plates in the presence of various concentrations of IL-2 or IL-7 at 37°C for 48 hours. [3H]Thymidine (1 μCi) was added into the culture 4 hours before harvesting. Cells were harvested with a cell harvester on a glass filter (Harvester 96; Tomtec, Hamden, CT). Radioactivity was determined by a liquid scintillation counter (1450 LSC & Luminescence Counter, PerkinElmer Life and Analytical Sciences, Waltham, MA).
Immunoprecipitation and immunoblotting
Antibodies used for immunoprecipitation and immunoblotting are as follows: Anti-Jak1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Jak3 (Upstate Biotechnology, Lake Placid, NY), anti-Akt (Cell Signaling Technology, Danvers, MA), anti-Shc (BD Transduction Laboratories, Lexington, KY), anti-phosphotyrosine (4G10; Upstate), anti-phospho-Akt (Thr308; Cell Signaling Technology), anti-phospho-Stat5 (Tyr694; Cell Signaling Technology) and anti-phospho-p44/42 mitogen-activated protein (MAP) kinase (Thr202/Tyr204; Cell Signaling Technology).
CTLL-2 and its derivatives were cultured in complete medium without IL-2 for 6 hours. These factor-starved cells were stimulated with saturating amounts of IL-2 (10 ng/mL) or IL-7 (100 ng/mL) for 10 minutes at 37°C. Cells were centrifuged and solubilized with lysis buffer (1% Triton X-100,50 mM Tris-Cl, 300 mM NaCl, and 5 mM EDTA) with protease inhibitor cocktail and phosphatase inhibitor cocktail (Sigma). After centrifugation, cell lysates were subjected to immunoprecipitation or sodium dodecyl sulfate (SDS)/polyacrylamide gel electrophoresis (PAGE) followed by Western blotting.
For immunoprecipitation, antibodies indicated in figures were incubated with 15 μl (pack) Protein A Sepharose 4 Fast Flow (GE Healthcare, Chalfont St. Giles, United Kingdom). After washing with lysis buffer, protein A Sepharose beads were further incubated with cell lysates prepared as above at 4°C overnight with gentle rotation. Immunoprecipitates were subjected to SDS/PAGE (10% gel) and electrophoretically transferred onto Immobilon-FL membrane (Millipore, Billerica, MA). After blocking with 3% bovine serum albumin (BSA) in Tris buffered saline/Tween 20 (TBS-T; 10 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween 20, pH 7.5), membranes were incubated with antibodies specified in figures in 1% BSA in TBS-T. After washing, membranes were further incubated with Alexa Fluor 680-conjugated anti-mouse or rabbit immunoglobulin (Invitrogen). Membranes were analyzed by Odyssey infrared imagine system (LI-COR Biosciences, Lincoln, NE).
Modulation of MAPK activity
2′-Amino-3′-methoxyflavone (PD98059; 5 μM) and 1,4-diamino-2,3-dicyano-1,4-bis(methylthio)butadiene (U0126; 5 μM) were added into the in vitro culture. Constitutive active form of MEK1 (Invitrogen) and siRNA-targeting MEK1 (Millipore) were cloned into the MSCV-IRES-GFP vector. To test gain-of-function or loss-of-function effects of the MEK/ERK pathway in hematopoiesis in vivo, HSCs (5 × 104, CD45.1) were retrovirally transduced with either active MEK1 or siRNA-MEK1 and were injected into sublethally irradiated RAG2−/− (CD45.2) mice. Peripheral blood was collected at the indicated time points in the figure after injection. Thymus, spleen, and bone marrow of the reconstituted mice were analyzed 6 weeks after injection. The effect of siRNA used in this study is shown in Figure S2.
Single cell clonal assay
The clonal assay was performed as described previously.40 In brief, single VCAM-1− MPPs were sorted into each well of 96-well plates with OP9 stromal cell layers by the automatic cell deposition unit on the FACSVantage SE (BD Biosciences). Cells were then cultured in the presence of SCF (50 ng/mL) and Flt3L (30 ng/mL) in the presence or absence of MAPK inhibitors. Two days later, IL-3 (10 ng/mL), GM-CSF (10 ng/mL), and IL-7 (10 ng/mL) were further added into the culture to support myeloid and lymphoid differentiation. After an additional 8 days spent in culture, each well was individually harvested by vigorous pipetting and analyzed by FACS.
Results
Signals via IL-2Rβ were necessary for lineage conversion of CLPs
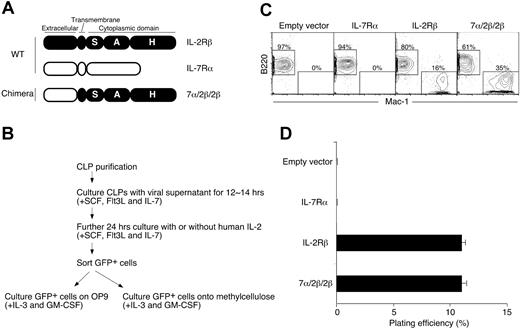
To examine whether unique signals from IL-2Rβ induce lineage conversion, we generated a chimeric receptor that was composed of the extracellular domain of IL-7Rα and the transmembrane and cytoplasmic domains of IL-2Rβ (7α/2β/2β; Figure 1A). Using retroviral gene transfer systems, we introduced wild-type (WT) IL-2Rβ, WT IL-7Rα, or 7α/2β/2β into CLPs. After introduction of these exogenous genes, we further stimulated CLPs for 24 hours with IL-2 (for IL-2Rβ) or IL-7 (for mock control, IL-7Rα, and 7α/2β/2β) (Figure 1B). We then sorted GFP+ cells and examined the myeloid differentiation potential of pre-cultured CLPs. As previously shown, even in the presence of cytokines that drive GM differentiation, CLPs expressing exogenous IL-7Rα differentiated into B220+ B cells but not Mac-1+ myeloid (or GM) cells26,42 (Figure 1C). In contrast, IL-7-stimulated 7α/2β/2β+ CLPs as well as IL-2–stimulated IL-2Rβ+ CLPs gave rise to myeloid cells (Figure 1C). Similar to the results in the stromal cell culture with OP9, IL-2–stimulated IL-2Rβ+ CLPs and IL-7–stimulated 7α/2β/2β+ CLPs formed GM colonies on methylcellulose at comparable plating efficiencies (Figure 1D). Because the transmembrane domain is not involved in signaling,43,44 these results suggest that the unique lineage conversion signals initiated by ectopic IL-2 stimulation are triggered by the intracellular region of IL-2Rβ.
IL-2Rβ cytoplasmic domain delivers lineage conversion signal in CLPs. (A) Schematic representation of receptors and their domains. Shown are WT IL-2Rβ (closed), WT IL-7Rα (open), and 7α/2β/2β. The chimeric receptor 7α/2β/2β is composed of the extracellular domain of IL-7Rα and the transmembrane and cytoplasmic domains of IL-2Rβ. The cytoplasmic tail of IL-2Rβ can be divided into 3 subdomains, S, A, and H regions. (B) Flow chart of experimental system. (C) Introduction of 7α/2β/2β chimera can initiate lineage conversion in CLPs in response to IL-7. CLPs were retrovirally transduced with empty vector, IL-7Rα, IL-2Rβ, and 7α/2β/2β. GFP+ cells were sorted and cultured on OP9 stromal cell layers in the presence of IL-3 and GM-CSF for 5 days. B220+ B-cell and Mac-1+ myeloid-cell readout was analyzed by FACS. Numbers shown are the percentage of total cells in the gates. B220+ cells from IL-2Rβ+ CLPs proliferated more than B220+ cells from 7α/2β/2β+ CLPs in this culture condition. (D) Myeloid differentiation potential of chimeric receptor-expressing CLPs was assessed in methylcellulose medium supplemented with IL-3 and GM-CSF. The plating efficiency was determined by enumerating the number of GM colonies formed after 5 to 7 days in the culture. Error bars represent standard deviation from the triplicate samples.
IL-2Rβ cytoplasmic domain delivers lineage conversion signal in CLPs. (A) Schematic representation of receptors and their domains. Shown are WT IL-2Rβ (closed), WT IL-7Rα (open), and 7α/2β/2β. The chimeric receptor 7α/2β/2β is composed of the extracellular domain of IL-7Rα and the transmembrane and cytoplasmic domains of IL-2Rβ. The cytoplasmic tail of IL-2Rβ can be divided into 3 subdomains, S, A, and H regions. (B) Flow chart of experimental system. (C) Introduction of 7α/2β/2β chimera can initiate lineage conversion in CLPs in response to IL-7. CLPs were retrovirally transduced with empty vector, IL-7Rα, IL-2Rβ, and 7α/2β/2β. GFP+ cells were sorted and cultured on OP9 stromal cell layers in the presence of IL-3 and GM-CSF for 5 days. B220+ B-cell and Mac-1+ myeloid-cell readout was analyzed by FACS. Numbers shown are the percentage of total cells in the gates. B220+ cells from IL-2Rβ+ CLPs proliferated more than B220+ cells from 7α/2β/2β+ CLPs in this culture condition. (D) Myeloid differentiation potential of chimeric receptor-expressing CLPs was assessed in methylcellulose medium supplemented with IL-3 and GM-CSF. The plating efficiency was determined by enumerating the number of GM colonies formed after 5 to 7 days in the culture. Error bars represent standard deviation from the triplicate samples.
IL-7R acquired lineage conversion ability by addition of the A region of IL-2Rβ to IL-7Rα
It has been suggested that different signaling pathways are initiated through distinct regions of the cytoplasmic tail of cytokine receptor subunits. For example, it has been demonstrated that distinct cytoplasmic regions of G-CSF receptor can initiate different functions.45-47 Therefore, we hypothesized that distinct regions of IL-2Rβ might activate unique signaling pathways that are necessary for lineage conversion. The cytoplasmic domain of the IL-2Rβ chain can be divided into 3 sub-domains based on their primary sequences (Figure 2A).5 The serine-rich (S) region contains a Janus kinase (Jak) 1 association site and is indispensable for triggering downstream signal transduction cascades via IL-2R.48-50 The acidic (A) region contains 4 tyrosine residues and is known to activate the MAPK pathway via Shc.51-54 The H region, which spans the C-terminal half of the cytoplasmic tail, has 2 tyrosine residues, both of which play crucial roles in Stat5 activation.5,55,56
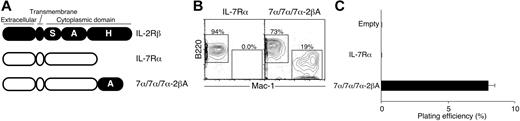
Lineage conversion ability of IL-2Rβ is transferable to IL-7Rα. (A) Diagram of chimeric receptors that are composed of IL-7Rα and subdomains of the IL-2Rβ cytoplasmic tail. The A region of IL-2Rβ was fused to the C terminus of IL-7Rα, resulting in 7α/7α/7α-2βA. Addition of the A region of IL-2Rβ to IL-7Rα enables lineage conversion activity of IL-7R as judged by differentiation readout on (B) OP9 stromal cell cultures and (C) methylcellulose cultures. (B) Percentages indicate total cells in the gates as described in Figure 1C. (C) Error bars represent standard deviation from triplicate samples.
Lineage conversion ability of IL-2Rβ is transferable to IL-7Rα. (A) Diagram of chimeric receptors that are composed of IL-7Rα and subdomains of the IL-2Rβ cytoplasmic tail. The A region of IL-2Rβ was fused to the C terminus of IL-7Rα, resulting in 7α/7α/7α-2βA. Addition of the A region of IL-2Rβ to IL-7Rα enables lineage conversion activity of IL-7R as judged by differentiation readout on (B) OP9 stromal cell cultures and (C) methylcellulose cultures. (B) Percentages indicate total cells in the gates as described in Figure 1C. (C) Error bars represent standard deviation from triplicate samples.
Although similar signaling pathways are activated via IL-2 and IL-7 receptors,29-31 a major difference lies in the A region of IL-2Rβ. The A region of IL-2Rβ serves as a docking site for the adaptor molecule Shc, whereas IL-7Rα contains no Shc binding site.36 In addition, we have shown that IL-2Rβ with an H region deletion can still activate latent myeloid differentiation in CLPs,26 suggesting that the A region itself may be sufficient to activate a specific pathway that leads to lineage conversion in CLPs. To clarify this issue, we tested whether the addition of the A region of IL-2Rβ to the cytoplasmic tail of IL-7Rα (7α/7α/7α-2βA, Figure 2A) could transfer the lineage conversion potential of IL-2R to IL-7R. After introduction of either WT IL-7Rα or 7α/7α/7α-2βA into CLPs using a retroviral system, we stimulated the cells with IL-7 and examined the myeloid differentiation potential of CLPs as described in Figure 1B. Although IL-7Rα+ CLPs did not give rise to Mac-1+ myeloid cells, 7α/7α/7α-2βA+ CLPs showed Mac-1+ myeloid cell differentiation in OP9 stromal cell cultures (Figure 2B). Likewise, 7α/7α/7α-2βA+ CLPs formed colonies on methylcellulose in the presence of IL-3 and GM-CSF (Figure 2C). The colony-forming cells in this culture showed immature myelocytic morphology, whereas the colonies from 7α/2β/2β+ CLPs in Figure 1D were composed of mature granulocytes and macrophages (data not shown). These data clearly demonstrate that the lineage conversion ability of IL-2R is transferable to IL-7R. They also suggest that the lineage conversion signals can be delivered via the A region of IL-2Rβ. Because it is not clear whether the immature myelocytic cells have potential to give rise to both granulocyte and macrophage lineages or either granulocyte or macrophage, we simply refer to myeloid cells derived from CLPs after lineage conversion as GM cells or myeloid cells hereafter in this article.
Addition of the IL-2Rβ A region to IL-7Rα did not affect the signal strength of IL-7R
In the course of this study, we found that proliferation mediated by IL-7 is much weaker than that mediated by IL-2. Although splenocytes activated with concanavalin A proliferated in response to both IL-2 and IL-7, the maximum [3H]thymidine incorporation of splenocytes in response to IL-7 was ∼25% of that of splenocytes stimulated by IL-2 (data not shown). Similar low growth promotion activity of IL-7 compared with IL-2 is observed in CTLL-2, a murine T-cell line, when it is ectopically transduced with IL-7Rα (Figure 3A,B). The proliferation rate in response to IL-7 was approximately 25% to 30% of the maximum response to IL-2 in these cells (Figure 3A). In contrast, the level of [3H]thymidine incorporation by 7α/2β/2β+ CTLL-2 in response to IL-7 was in the same range as stimulation by IL-2 (Figure 3A).
Addition of IL-2Rβ sub-domains to IL-7Rα does not alter the signal strength. (A) CTLL-2 cells (left) and their derivatives that express IL-7Rα (middle) or 7α/2β/2β (right) were washed and then cultured in the presence of various concentrations of IL-2 (○) or IL-7 (•) for 48 hours. [3H]thymidine was added during the last 4 hours of culture. Each point represents the mean value of triplicate samples. (B) Staining of CTLL-2 and its derivatives with anti–IL-7Rα antibodies (—) to determine the receptor surface expression level. Negative staining levels with isotype control are also shown (– – –). (C) Monitoring proliferation response upon cytokine stimulation by [3H]thymidine incorporation. CTLL-2 and its derivatives were subjected to proliferation assays in response to saturating dosage of IL-7 (100 ng/mL) as described in “Materials and methods, Proliferation assays.” There was no significant difference between IL-7Rα and 7α/7α/7α-2βA. Error bars represent standard deviation from triplicate samples. (D) Phosphorylation of signaling molecules in CTLL-2 transfectants in response to IL-7. Cells were stimulated with saturating dosage of IL-7 (100 ng/mL) for 10 minutes and then lysed. IL-2 stimulated and nonstimulated IL-7Rα+ CTLL-2 cells were applied as positive and negative controls, respectively. Immunoprecipitates and whole-cell lysates (WCL) were resolved by SDS-PAGE and transferred to membranes. The membranes were probed with anti-pTyr, and anti-pStat5 antibodies or probed with anti-Stat5 antibodies for loading controls. The amount of immunoprecipitated protein was determined by blotting with anti-Jak1, and anti-Jak3.
Addition of IL-2Rβ sub-domains to IL-7Rα does not alter the signal strength. (A) CTLL-2 cells (left) and their derivatives that express IL-7Rα (middle) or 7α/2β/2β (right) were washed and then cultured in the presence of various concentrations of IL-2 (○) or IL-7 (•) for 48 hours. [3H]thymidine was added during the last 4 hours of culture. Each point represents the mean value of triplicate samples. (B) Staining of CTLL-2 and its derivatives with anti–IL-7Rα antibodies (—) to determine the receptor surface expression level. Negative staining levels with isotype control are also shown (– – –). (C) Monitoring proliferation response upon cytokine stimulation by [3H]thymidine incorporation. CTLL-2 and its derivatives were subjected to proliferation assays in response to saturating dosage of IL-7 (100 ng/mL) as described in “Materials and methods, Proliferation assays.” There was no significant difference between IL-7Rα and 7α/7α/7α-2βA. Error bars represent standard deviation from triplicate samples. (D) Phosphorylation of signaling molecules in CTLL-2 transfectants in response to IL-7. Cells were stimulated with saturating dosage of IL-7 (100 ng/mL) for 10 minutes and then lysed. IL-2 stimulated and nonstimulated IL-7Rα+ CTLL-2 cells were applied as positive and negative controls, respectively. Immunoprecipitates and whole-cell lysates (WCL) were resolved by SDS-PAGE and transferred to membranes. The membranes were probed with anti-pTyr, and anti-pStat5 antibodies or probed with anti-Stat5 antibodies for loading controls. The amount of immunoprecipitated protein was determined by blotting with anti-Jak1, and anti-Jak3.
This result prompted us to examine IL-7–mediated cell growth promotion through 7α/7α/7α-2βA, because it is possible that addition of the A region of IL-2Rβ allows IL-7R to transduce stronger signals than WT IL-7R complexes. If so, the difference between IL-2R and IL-7R in their ability to induce lineage conversion in CLPs may be simply explained by a difference of signal strength. However, the proliferation rate of 7α/7α/7α-2βA CTLL-2 cells was the same as that of IL-7Rα+ CTLL-2 as shown in Figure 3C. Surface expression levels of WT IL-7Rα and chimeric receptors were comparable (Figure 3B). In addition, the phosphorylation levels of immediate downstream signaling molecules of IL-2R and IL-7R, such as Jak1, Jak3, and Stat5, correlated with growth promotion activity but not with lineage conversion ability (Figure 3D). These results suggest that signal strength cannot explain the ability of 7α/7α/7α-2βA+ to initiate lineage conversion. Therefore, signals triggered via the A region of IL-2Rβ most likely contain a unique signal that is absent in IL-7R signaling and is necessary for lineage conversion.
Y338 of IL-2Rβ was indispensable for A region–mediated lineage conversion signal
To identify the required signals for lineage conversion in CLPs, we first determined which part of the A region was necessary to initiate lineage conversion. We separated the A region into 2 halves, amino acids (a.a.) 313-353 and 354-382, and fused each subregion to the C terminus of IL-7Rα (Figure 4A). After introduction of each construct to CLPs, we examined IL-7–mediated lineage conversion activity as in Figure 2. We found that the proximal half (a.a. 313-353) but not the distal half of the A region is sufficient to initiate lineage conversion in CLPs (Figure 4B).
The proximal region and tyrosine residue 338 of IL-2Rβ A region are crucial for lineage conversion. (A) Illustration of deletion chimeric receptors. IL-2Rβ A region was divided into 2 sub-regions (a.a. 313-353 [proximal] and a.a. 354-382 [distal]). (B) The chimeric receptors shown in panel A were retrovirally introduced into CLPs. The lineage conversion ability of each chimeric receptor was examined by myeloid colony forming ability in methylcellulose culture in the presence of IL-3 and GM-CSF. (C) Diagram of tyrosine-to-phenylalanine mutants. The individual tyrosine (Y) residues within IL-2Rβ A region were mutated into phenylalanine (F). (D) Lineage conversion ability was assessed with the methylcellulose culture as described for panel B. (B,D) Error bars represent standard deviation from triplicate samples.
The proximal region and tyrosine residue 338 of IL-2Rβ A region are crucial for lineage conversion. (A) Illustration of deletion chimeric receptors. IL-2Rβ A region was divided into 2 sub-regions (a.a. 313-353 [proximal] and a.a. 354-382 [distal]). (B) The chimeric receptors shown in panel A were retrovirally introduced into CLPs. The lineage conversion ability of each chimeric receptor was examined by myeloid colony forming ability in methylcellulose culture in the presence of IL-3 and GM-CSF. (C) Diagram of tyrosine-to-phenylalanine mutants. The individual tyrosine (Y) residues within IL-2Rβ A region were mutated into phenylalanine (F). (D) Lineage conversion ability was assessed with the methylcellulose culture as described for panel B. (B,D) Error bars represent standard deviation from triplicate samples.
Next, we determined whether the tyrosine residues in the A region are required for lineage conversion. We constructed a series of point mutants in which tyrosine (Y) residues of the A region of 7α/7α/7α-2βA were changed to phenylalanine (F) residues (Figure 4C). As shown in Figures 4D, 7α/7α/7α-2βA(F338/3Y)+ and 7α/7α/7α-2βA(F338/3F)+ CLPs did not undergo lineage conversion after IL-7 stimulation. In contrast, IL-7–stimulated 7α/7α/7α-2βA(Y338/3F)+ CLPs formed colonies on methylcellulose in the presence of cytokines that support GM differentiation (Figure 4D). These data suggest that Y338 in the proximal half of the A region is indispensable for the initiation of lineage conversion through IL-2Rβ.
Shc was a key molecule in lineage conversion signals via the A region of IL-2Rβ
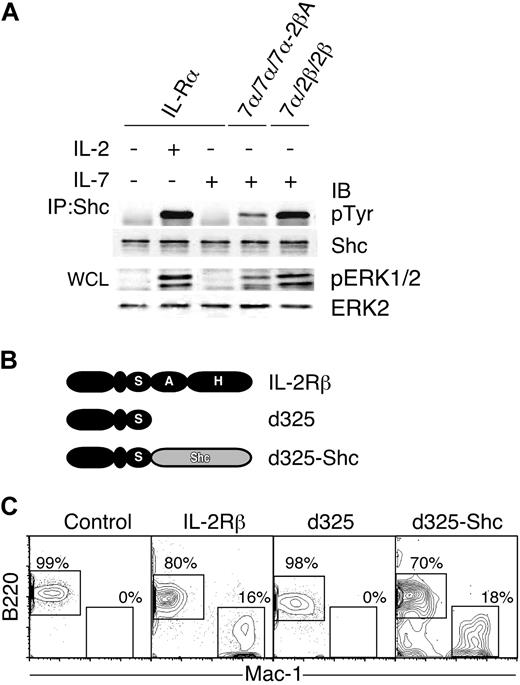
Shc is known to be involved in signal transduction via IL-2R51-54 but not IL-7R.36 In addition, Y338, the docking site for Shc, was necessary for A region-mediated lineage conversion as shown in Figure 4. Therefore, we hypothesized that Shc might be a key molecule for triggering lineage conversion mediated by the A region of IL-2Rβ. To explore this possibility, we first examined the phosphorylation level of Shc in CTLL-2 cells. As shown in Figure 5A, Shc phosphorylation was observed in 7α/7α/7α-2βA+ CTLL-2 cells after IL-7 stimulation, whereas no IL-7–mediated Shc phosphorylation was detected in IL-7Rα+ CTLL-2 cells. This suggests that addition of the A region to IL-7Rα confers Shc activation potential to IL-7R. It has been shown that phospho-Shc activates the MAPK pathway through Ras.53,57 Indeed we observed ERK1 and ERK2 phosphorylation in 7α/7α/7α-2βA+ but very little or no phosphorylation in IL-7Rα+ CTLL-2 cells after IL-7 stimulation (Figure 5A). These results demonstrate that IL-7R acquires higher MAPK activity through the addition of the IL-2Rβ A region.
Involvement of Shc in IL-2–mediated lineage conversion in CLPs. (A) CTLL-2 transfectants were stimulated with cytokines as indicated in the figure. Cell lysates were subjected to SDS-PAGE or immunoprecipitation. Immunoprecipitates were resolved by SDS-PAGE and transferred to membranes. Membranes were probed with anti-pTyr and anti-Shc antibodies. Whole-cell lysates (WCL) were used for the detection of pERK1/2 and ERK2. (B) Schematic depiction of IL-2Rβ mutants. The d325 lacks both A and H regions. A full-length Shc is fused to the C terminal of the d325 mutant, resulting in d325-Shc. (C) IL-2Rβ mutants were introduced into CLPs by retroviral transduction. GFP+ cells were sorted and cultured on OP9 stromal layers in the presence of IL-3 and GM-CSF. At day 5 of culture, cells were harvested and subjected to FACS analysis. Percentages indicate total cells in the gates as described in Figure 1C.
Involvement of Shc in IL-2–mediated lineage conversion in CLPs. (A) CTLL-2 transfectants were stimulated with cytokines as indicated in the figure. Cell lysates were subjected to SDS-PAGE or immunoprecipitation. Immunoprecipitates were resolved by SDS-PAGE and transferred to membranes. Membranes were probed with anti-pTyr and anti-Shc antibodies. Whole-cell lysates (WCL) were used for the detection of pERK1/2 and ERK2. (B) Schematic depiction of IL-2Rβ mutants. The d325 lacks both A and H regions. A full-length Shc is fused to the C terminal of the d325 mutant, resulting in d325-Shc. (C) IL-2Rβ mutants were introduced into CLPs by retroviral transduction. GFP+ cells were sorted and cultured on OP9 stromal layers in the presence of IL-3 and GM-CSF. At day 5 of culture, cells were harvested and subjected to FACS analysis. Percentages indicate total cells in the gates as described in Figure 1C.
Next, we tested whether there is a direct connection between Shc activation and lineage conversion in CLPs. For this purpose, we used the d325-Shc construct,37 which is composed of the IL-2Rβ d325 mutant and the entire Shc (Figure 5B). The d325 mutant is an IL-2Rβ mutant in which the entire A and H regions have been deleted.37,58 The d325+ CLPs did not give rise to myeloid cells by IL-2 stimulation (Figure 5C), supporting our previous observation that the presence of either the A or H region is necessary for initiation of lineage conversion after IL-2 stimulation.26 In contrast, IL-2–stimulated d325-Shc+ CLPs gave rise to Mac-1+ myeloid cells in stromal cell cultures (Figure 5C) and formed myeloid colonies on methylcellulose in the presence of IL-3 and GM-CSF at a frequency of 3.25% (± 0.14%). These data directly demonstrate that Shc plays a critical role in the IL-2Rβ A region–mediated lineage conversion signal.
MEK/ERK activation was necessary for myeloid lineage commitment
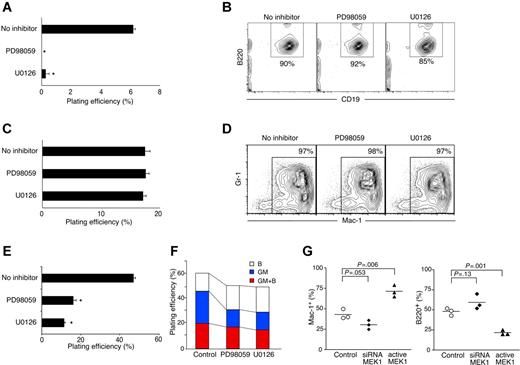
We examined whether lineage conversion of CLPs mediated by the A region of IL-2Rβ can be blocked by the presence of inhibitors for the MAPK pathway. For this purpose, we used the dH mutant of IL-2Rβ.5,26 In this mutant, the IL-2Rβ H region is deleted, whereas the A region, which serves as a binding site for Shc, is intact. After introduction of the dH mutant into CLPs, we stimulated these cells with IL-2 in the presence or absence of PD98059 and U0126, which are inhibitors specific for MEK/ERK activation in the MAPK pathway.59,60 The dH+ CLPs were sorted and plated onto methylcellulose with IL-3 and GM-CSF. As shown in Figure 6A, lineage conversion of CLPs initiated by IL-2 stimulation was almost completely blocked by the MEK/ERK inhibitors. In addition, the dH+ CLPs that had been precultured in the presence of the MEK/ERK inhibitors but in the absence of IL-2 stimulation gave rise to B220+CD19+ B cells in the stromal cell culture with IL-7 (Figure 6B). Therefore, the presence of MEK/ERK inhibitors during the preculture does not affect the normal differentiation potential of CLPs, but it specifically blocks IL-2R–induced lineage conversion.
The role of MAPK pathway in lineage conversion and myeloid differentiation. (A) The IL-2RβdH mutant, which has the complete A region with H region removed, was introduced to CLPs. Double-sorted CLPs were first cultured with retrovirus containing supernatant in the presence of SCF, Flt3L, IL-7, and 5 μM PD98059 or 5 μM U0126 for 24 hours. IL-2 was then added into culture to induce lineage conversion, and cells were further cultured for an additional 24 hours. GFP+ cells were sorted onto methylcellulose medium and cultured in the presence of IL-3 and GM-CSF. Plating efficiency was determined by enumerating GM colonies after 5 days of culture. (B) To determine the B-cell developmental potential, CLPs were cultured with retroviral supernatants in the presence of SCF, Flt3L, IL-7, and MEK/ERK inhibitors for 48 hours. GFP+ cells were sorted and cocultured on an OP9 stromal layer with SCF, Flt3L and IL-7. B220+CD19+ B-cell readout was examined by FACS analysis at day 7. (C) CMPs were cultured in the presence of GM-CSF, IL-3, and SCF with MEK/ERK inhibitors as described for panel B. Plating efficiency was determined by counting GM colonies after 5 days of culture. (D) CMPs were sorted and cultured on OP9 stromal layers in the presence of MEK/ERK inhibitors. Fresh medium with inhibitors was added every 3 days during the culture period. The presence of Mac-1+Gr-1+ myeloid cells was examined by FACS analysis. (E) MEK/ERK activation is crucial in myeloid commitment from HSCs. Flt3−c-KithiLin−/loSca-1+ HSCs were sorted and cultured in the presence of MEK/ERK inhibitors as described. GM colony forming ability of HSCs was examined by enumerating colonies in methylcellulose medium supplemented with IL-3 and GM-CSF at day 6 of the culture. (F) Inhibition of MAPK activity represses myeloid cell readout from MPPs. The presence of both Mac-1+ and B220+ cells from single VCAM-1−Flt3+ MPPs was defined as bipotent (GM + B; red), whereas readout of either Mac-1+ or B220+ was defined as single lineage, GM only (blue), or B only (open). Bipotent and single lineage readout was examined in the absence (control) or presence of MEK/ERK inhibitors (PD98059 and U0126). (G) Loss-of-function and gain-of-function effect of MEK1 on lineage decision. HSCs that had been transduced with siRNA targeting MEK1 or with a constitutively active form of MEK1 were injected into RAG2−/− hosts. Peripheral blood was collected at week 4 and percentage of GM (Mac-1+) and B cells (B220+) in the GFP+ donor fraction was analyzed. T cells were under the detectable level in the blood at the time points we examined (2-6 weeks after injection). However, we could identify similar numbers of TCRβ+ T cells in the spleen at week 6 (data not shown). (A,C,E) Error bars represent standard deviation from triplicate samples. (B,D) Percentages indicate total cells in the gates as described in Figure 1C. (G) Horizontal bars represent the mean value from 3 mice.
The role of MAPK pathway in lineage conversion and myeloid differentiation. (A) The IL-2RβdH mutant, which has the complete A region with H region removed, was introduced to CLPs. Double-sorted CLPs were first cultured with retrovirus containing supernatant in the presence of SCF, Flt3L, IL-7, and 5 μM PD98059 or 5 μM U0126 for 24 hours. IL-2 was then added into culture to induce lineage conversion, and cells were further cultured for an additional 24 hours. GFP+ cells were sorted onto methylcellulose medium and cultured in the presence of IL-3 and GM-CSF. Plating efficiency was determined by enumerating GM colonies after 5 days of culture. (B) To determine the B-cell developmental potential, CLPs were cultured with retroviral supernatants in the presence of SCF, Flt3L, IL-7, and MEK/ERK inhibitors for 48 hours. GFP+ cells were sorted and cocultured on an OP9 stromal layer with SCF, Flt3L and IL-7. B220+CD19+ B-cell readout was examined by FACS analysis at day 7. (C) CMPs were cultured in the presence of GM-CSF, IL-3, and SCF with MEK/ERK inhibitors as described for panel B. Plating efficiency was determined by counting GM colonies after 5 days of culture. (D) CMPs were sorted and cultured on OP9 stromal layers in the presence of MEK/ERK inhibitors. Fresh medium with inhibitors was added every 3 days during the culture period. The presence of Mac-1+Gr-1+ myeloid cells was examined by FACS analysis. (E) MEK/ERK activation is crucial in myeloid commitment from HSCs. Flt3−c-KithiLin−/loSca-1+ HSCs were sorted and cultured in the presence of MEK/ERK inhibitors as described. GM colony forming ability of HSCs was examined by enumerating colonies in methylcellulose medium supplemented with IL-3 and GM-CSF at day 6 of the culture. (F) Inhibition of MAPK activity represses myeloid cell readout from MPPs. The presence of both Mac-1+ and B220+ cells from single VCAM-1−Flt3+ MPPs was defined as bipotent (GM + B; red), whereas readout of either Mac-1+ or B220+ was defined as single lineage, GM only (blue), or B only (open). Bipotent and single lineage readout was examined in the absence (control) or presence of MEK/ERK inhibitors (PD98059 and U0126). (G) Loss-of-function and gain-of-function effect of MEK1 on lineage decision. HSCs that had been transduced with siRNA targeting MEK1 or with a constitutively active form of MEK1 were injected into RAG2−/− hosts. Peripheral blood was collected at week 4 and percentage of GM (Mac-1+) and B cells (B220+) in the GFP+ donor fraction was analyzed. T cells were under the detectable level in the blood at the time points we examined (2-6 weeks after injection). However, we could identify similar numbers of TCRβ+ T cells in the spleen at week 6 (data not shown). (A,C,E) Error bars represent standard deviation from triplicate samples. (B,D) Percentages indicate total cells in the gates as described in Figure 1C. (G) Horizontal bars represent the mean value from 3 mice.
We further examined whether the growth of myeloid progenitors is likewise affected by MEK/ERK inhibitors. We cultured common myeloid progenitors (CMPs)39 on methylcellulose containing IL-3 and GM-CSF in the presence of PD98059 or U0126. After the culture, we observed comparable numbers of colonies in either the presence or absence of these inhibitors (Figure 6C). In addition, the size of the colonies in both groups was comparable (data not shown). Furthermore, CMPs gave rise to Gr-1+Mac-1+ GM cells on OP9 stromal cell layers in the presence of PD98059 and U0126 (Figure 6D), suggesting that MEK/ERK activity is not crucial for the proliferation of myeloid progenitors. Therefore, the MEK/ERK pathway activated by Shc through its association with the A region of IL-2Rβ plays an important role in the initiation of lineage conversion in CLPs but not in the growth and differentiation of CMPs.
Finally, we tested whether the MAPK pathway is necessary for GM lineage commitment in physiologic hematopoiesis. For this purpose, we examined the GM colony-forming activity of HSCs in the presence or absence of MEK/ERK inhibitors. As shown in Figure 6E, the GM colony number was decreased by approximately 60% in the presence of PD98059 and U0126. Because these 2 inhibitors had no inhibitory effect on myeloid cell survival or proliferation as shown in Figure 6C,D, the data shown here suggest that MAPK, specifically the MEK/ERK pathway, plays a role in GM lineage commitment from HSCs.
We further examined a potential role of the MEK/ERK pathway in lymphocyte development. We cultured single VCAM-1− MPP cells, which have not committed to either the myeloid or the lymphoid lineage and can give rise to Mac-1+ GM cells and B cells in in vitro stromal cell cultures40,41 (Figure 6F). In the presence of PD98059 or U0126, the plating efficiency of these cells was slightly reduced. Nevertheless, the frequency of GM-cell readout was clearly reduced in the presence of PD98059 or U0126 (Figure 6F). On the contrary, B-cell readout frequency was increased in the presence of the inhibitors, suggesting that the effect of PD98059 and U0126 in lymphoid lineage commitment is minimal or nonexistent. More importantly, some B-cell readout might occur as compensation for the reduced GM lineage commitment incurred by blocking the MEK/ERK pathway.
We also examined the effect of constitutive activation of MEK as well as knockdown of MEK by siRNA on hematopoiesis from HSCs in vivo. Similar to the result of in vitro cultures with MAPK inhibitors, HSCs with siRNA-targeting MEK gave rise to Mac-1+ myeloid cells less efficiently than control HSCs (Figure 6G). It is noteworthy that the mice with HSCs that had been transduced with active MEK had more Mac-1+ cells than the mice with control HSCs. The effect of siRNA for MEK in T-cell development was not obvious when we examined the spleen after 6 weeks of injection, whereas only a few T cells were observed in active MEK1 reconstituted animal (data not shown). Taken together, these data strongly imply that the MEK/ERK pathway plays an important role in myeloid differentiation, especially GM lineage commitment, not only in ectopic IL-2–driven lineage conversion in CLPs but also in physiologic hematopoiesis.
Discussion
Emerging evidence suggests that the MEK/ERK pathway is important for differentiation of various types of cells. The requirement of the MEK/ERK pathway has been shown in the differentiation of neuronal cells,61 myoblasts,62 and cells in the visual cortex.63 In the hematopoietic system, the MEK/ERK pathway has been shown to be crucial for thymocyte transition from double-negative to double-positive stage,64 as well as for megakaryocyte and erythrocyte differentiation.65,66 In addition, the MEK/ERK pathway has been implicated in myeloid differentiation in cell lines.67,68 In this article, we demonstrate that the MAPK pathway plays a critical role in ectopic IL-2R-mediated lineage conversion in CLPs. It is noteworthy that MEK/ERK activity, as part of MAPK pathways, seems to be critical for GM lineage commitment even in normal hematopoiesis.
We found that the involvement of Shc was the critical determinant for enabling lineage conversion in IL-2R (Figure 5). Erythropoietin receptor (EpoR) can also recruit Shc to perform its biologic function.69,70 However, ectopic Epo stimulation cannot initiate lineage conversion in CLPs.26 It has been demonstrated that CTLL-2 cells with exogenous EpoR cannot proliferate in response to Epo unless additional ectopic v-Ki-Ras or the constitutively activated form of MEK1, as well as excess amount of Jak2, are expressed.71-73 Therefore the usage of Shc by different cytokine receptors may lead to different outcomes in certain types of cells.
Using various myeloid cell lines, the involvement of the MAPK pathway in myeloid maturation has been implicated.67,68,74 However, it was not clear whether the MAPK pathway plays a role in myeloid lineage commitment. We show here that GM differentiation potential from myeloid committed progenitors, CMPs, was not affected by MEK/ERK inhibitors (Figure 6C,D). Therefore, the MEK/ERK pathway is dispensable for differentiation and proliferation once the cells are committed to the myeloid lineage. However, GM differentiation from HSCs (Figure 6E) and VCAM-1−Flt3+ MPPs (Figure 6F) was significantly lower in the presence of PD98059 and U0126. In addition, active MEK1 promotes GM cell differentiation from HSCs in vivo whereas siRNA-MEK1 inhibits it (Figure 6G). Hence, MEK/ERK activity plays an important role in GM lineage differentiation, especially at the onset of GM lineage commitment.
We reported recently that IL-2 stimulation through exogenously expressed IL-2R initiates CCAAT/enhancer-binding protein (C/EBPα) expression in CLPs.38 Because introduction of C/EBPα is sufficient to induce myeloid cell development from CLPs, C/EBPα might be a major target of IL-2R–mediated lineage conversion signals.38 Our preliminary data suggest that IL-2–mediated C/EBPα up-regulation is blocked in the presence of MEK/ERK inhibitors (data not shown). Therefore, the MAPK pathway may directly mediate C/EBPα expression in CLPs after IL-2 stimulation through ectopic IL-2R. The mechanism of how the MAPK pathway affects the expression of C/EBPα is a question for further investigation.
In contrast to the A region, H region-mediated lineage conversion signals are not clear. As we reported previously, the dA mutant, in which the A region is deleted from IL-2Rβ, can still initiate lineage conversion in CLPs.26 Stat5 is the only known signaling component that has been identified to associate with the IL-2Rβ H region.29 However, Stat5 is phosphorylated not only by IL-2 but also IL-7 stimulation, implying that some unidentified molecules other than Stat5 may be involved in signaling during H region-mediated lineage conversion. As we have shown here for Shc and the A region of IL-2Rβ, it is intriguing to identify what molecule(s) can mediate lineage conversion signals in CLPs associate with the H region of IL-2Rβ.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Brad Nelson for cDNAs of d325-Shc. We also thank Weiguo Zhang for allowing access to LI-COR odyssey scanner. We appreciate Anne Lai and Alexis Dunkle for critically reading the manuscript.
This work was supported by the Duke Stem Cell Research Program Annual Award, and National Institutes of Health grants AI056123 and CA098129 to M.K. M.K. was a scholar of the Sidney Kimmel Foundation for Cancer Research.
National Institutes of Health
Authorship
Contribution: C.-L. H. performed the research and wrote the manuscript; K.K. designed the research; and M.K. designed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr. Motonari Kondo, Department of Immunology, Duke University Medical Center, 3010 Research Dr, Durham, NC 27710; e-mail: motonari.kondo@duke.edu.

![Figure 3. Addition of IL-2Rβ sub-domains to IL-7Rα does not alter the signal strength. (A) CTLL-2 cells (left) and their derivatives that express IL-7Rα (middle) or 7α/2β/2β (right) were washed and then cultured in the presence of various concentrations of IL-2 (○) or IL-7 (•) for 48 hours. [3H]thymidine was added during the last 4 hours of culture. Each point represents the mean value of triplicate samples. (B) Staining of CTLL-2 and its derivatives with anti–IL-7Rα antibodies (—) to determine the receptor surface expression level. Negative staining levels with isotype control are also shown (– – –). (C) Monitoring proliferation response upon cytokine stimulation by [3H]thymidine incorporation. CTLL-2 and its derivatives were subjected to proliferation assays in response to saturating dosage of IL-7 (100 ng/mL) as described in “Materials and methods, Proliferation assays.” There was no significant difference between IL-7Rα and 7α/7α/7α-2βA. Error bars represent standard deviation from triplicate samples. (D) Phosphorylation of signaling molecules in CTLL-2 transfectants in response to IL-7. Cells were stimulated with saturating dosage of IL-7 (100 ng/mL) for 10 minutes and then lysed. IL-2 stimulated and nonstimulated IL-7Rα+ CTLL-2 cells were applied as positive and negative controls, respectively. Immunoprecipitates and whole-cell lysates (WCL) were resolved by SDS-PAGE and transferred to membranes. The membranes were probed with anti-pTyr, and anti-pStat5 antibodies or probed with anti-Stat5 antibodies for loading controls. The amount of immunoprecipitated protein was determined by blotting with anti-Jak1, and anti-Jak3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/5/10.1182_blood-2007-02-071761/6/m_zh80180706670003.jpeg?Expires=1769095278&Signature=3BJLCxDk2HyGcVlO244ldgtep096zjuguLhSdCB6cvpmZouZG2GzXVD6jf4SPHF6lnzP3vAXGoSNTMEjapBDOnNb47PtoHTOveK2xmOSYrQ6buQFNqOdJ2oq1VtMP3Dkp~N6ORTYqlImDq5yuvsSW5YbCsnrofl-mjbm3tEd15-CU3DVODfrUKxdDPSEDBeap6~ZReCQlaMQETgNY1P085MsPxn7df3zaFawrfvoUfL1VRdcRWk312d4iW84x~En93zJHgUY1m3X-55FM-UnT~hwTfTuqIaoPh-onsuCEcs2Ns1PTcT5dWkA5O2-8mrYinln10LnavVfrG8YqbfndQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. The proximal region and tyrosine residue 338 of IL-2Rβ A region are crucial for lineage conversion. (A) Illustration of deletion chimeric receptors. IL-2Rβ A region was divided into 2 sub-regions (a.a. 313-353 [proximal] and a.a. 354-382 [distal]). (B) The chimeric receptors shown in panel A were retrovirally introduced into CLPs. The lineage conversion ability of each chimeric receptor was examined by myeloid colony forming ability in methylcellulose culture in the presence of IL-3 and GM-CSF. (C) Diagram of tyrosine-to-phenylalanine mutants. The individual tyrosine (Y) residues within IL-2Rβ A region were mutated into phenylalanine (F). (D) Lineage conversion ability was assessed with the methylcellulose culture as described for panel B. (B,D) Error bars represent standard deviation from triplicate samples.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/5/10.1182_blood-2007-02-071761/6/m_zh80180706670004.jpeg?Expires=1769095278&Signature=1eqlt780x8wmwYr6hEsE9Kur5HpVLouYTV52fn-NviE4wqQoIOkvP0RrIeFf8eLTO5hvURMq7K4vzzBiXbLsABbFCtxJhbFohkkRo0roxaAiWRy74z5-eJdbhlzQw6wIbzzYgFgcAdm0qfJjXjA3XPmQO3ktQOgO0uV7wVbTmj37LHl4ZPHUShnP7UiYa3JcWZ~tpgknk9U5gpZPjH2FECXIBoXLdPLEz7r68kW1SDoxGZKq4mAQW1g8X3mcRgptTpSWkHb71FelmP5GV8UymIvpH2kKEji~jEhe24E5jkVZfvg7UaUb8qJNBrVP8No9UwYOMug87iMVjkCcplCd2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal