The article by Kubach and colleagues in this issue of Blood includes not 1, but 2, intriguing and rather unexpected findings. As its title suggests, the authors compared expression patterns in CD4+CD25+ regulatory T cells (Treg cells) with those in both resting and activated CD4+CD25− T-cell populations, and found prominent differential expression of the carbohydrate binding protein, galectin-10.
This first result was surprising enough, as galectin-10, better known as Charcot-Leyden crystal (CLC) protein, has long been considered an eosinophil/basophil-specific protein, and a unique marker of eosinophilic inflammation in peripheral tissues.1,2 As a second surprising result, the authors demonstrate that intracytoplasmic galectin-10 expression is not only necessary for limiting the proliferation of CD4+CD25+ Treg cells, but that it is also crucial for the Treg-cell–mediated suppression of cocultured CD4+ T cells.
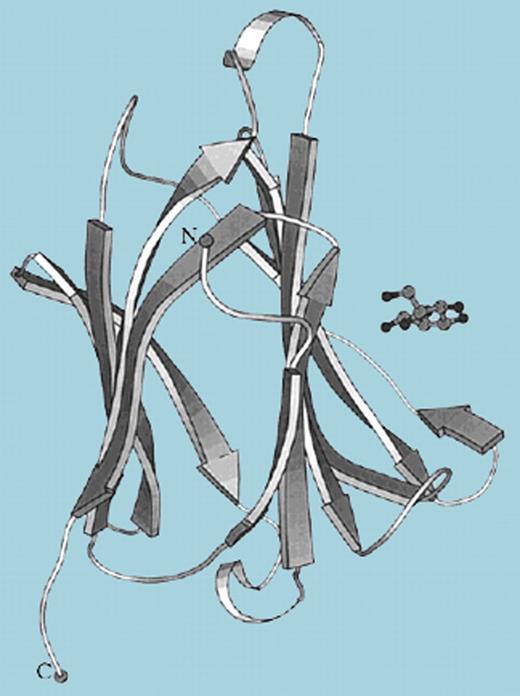
For some historical perspective: galectin-10/CLC is a well-known major component of human eosinophilic leukocytes, comprising 7% to 10% of the total eosinophil protein, and localized primarily to a small cytoplasmic granule fraction.3 CLC was first described in 1853 by J. M. Charcot and colleagues, who characterized the crystalline deposits of this protein in the spleen of a leukemic patient, and again in 1872 by Leyden in his studies of sputum from asthmatics.4 Although initially identified as a lysophospholipase, the complete amino acid sequence5 revealed significant homology with S-type animal lectins, now known as galectins, a multiprotein superfamily with specific affinity for galactose-containing carbohydrate moieties and characterized roles in tumor immune surveillance and inflammation. Yet, despite sequence homology, upon solving the crystal structure, Swaminathan and colleagues6 revealed the structural basis for galectin-10/CLC's primary affinity, which is for mannose, rather than the beta-galactoside sugars recognized by the galectins (see figure).
Ribbon representation of galectin-10/Charcot-Leyden crystal protein structure. Mannose (molecule at right) interacts with His 55, Asn 65, Trp 72, and Gln 75 residues within the carbohydrate recognition domain. Reprinted with permission from Swaminathan et al.6
Ribbon representation of galectin-10/Charcot-Leyden crystal protein structure. Mannose (molecule at right) interacts with His 55, Asn 65, Trp 72, and Gln 75 residues within the carbohydrate recognition domain. Reprinted with permission from Swaminathan et al.6
At this moment, it is difficult to apply much of the pre-existing knowledge regarding galectin-10/CLC to the findings presented in this manuscript. One can certainly envision a role for a secreted mannose-binding protein and mannose-containing ligand/receptor in promoting growth suppression, but the authors note that galectin-10/CLC remains intracytoplasmic and is apparently not secreted or released from CD4+CD25+ Treg cells under the experimental conditions described. As such, we have no real sense as to what the suppressive mechanism might be. Is mannose binding actually involved in the observed suppressive effects at all? What other molecules might interact with galectin-10/CLC, and how might this information ultimately be transmitted in order to suppress proliferation of cocultured CD4+ T cells? Additionally, as there is no evidence for a gene or genes encoding galectin-10/CLC in the mouse genome, what protein might promote this critical function in rodent Treg cells?
Equally intriguing, what precisely do these findings reveal about galectin-10/CLC and its role in the biology of the eosinophil? Eosinophils are enigmatic cells whose role in innate immunity remains unclear.7 Might human eosinophils, via abundant expression of galectin-10/CLC, also inhibit proliferation and function of CD4+ T cells? Jacobsen et al8 recently reviewed the literature on the immunomodulatory properties of eosinophils, which includes a variety of observations on the impact of these cells, presumably via antigen presentation and cytokine secretion, on CD4+ T-cell function. Given the findings presented here, the role of galectin-10/CLC in modulating human eosinophil-CD4+ T-cell interactions seems worthy of further exploration.
This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID)Division of Intramural Research.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal