Abstract
Perforin (PRF), a pore-forming protein expressed in cytotoxic lymphocytes, plays a key role in immune surveillance and immune homeostasis. The A91V substitution has a prevalence of 8% to 9% in population studies. While this variant has been suspected of predisposing to various disorders of immune homeostasis, its effect on perforin's function has not been elucidated. Here we complemented, for the first time, the cytotoxic function of perforin-deficient primary cytotoxic T lymphocytes (CTLs) with wild-type (hPRF-WT) and A91V mutant (hPRF-A91V) perforin. The cytotoxicity of hPRF-A91V–expressing cells was about half that of hPRF-WT–expressing counterparts and coincided with a moderate reduction in hPRF-A91V expression. By contrast, the reduction in cytotoxic function was far more pronounced (more than 10-fold) when purified proteins were tested directly on target cells. The A91V substitution can therefore be manifested by abnormalities at both the lymphocyte (presynaptic) and target cell (postsynaptic) levels. However, the severe intrinsic defect in activity can be partly rescued by expression in the physiological setting of an intact CTL. These findings provide the first direct evidence that hPRF-A91V is functionally abnormal and provides a rationale for why it may be responsible for disordered immune homeostasis if inherited with another dysfunctional perforin allele.
Introduction
Perforin (PRF; encoded by the PRF1 gene) is a pore-forming protein stored in secretory granules of cytotoxic lymphocytes (CLs) comprising cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells. CLs destroy virus-infected or -transformed target cells predominantly through the “granule exocytosis” mechanism, in which the contents of cytotoxic secretory granules are released into the synapse formed between the CL and the target cell. There, perforin synergizes with other granule components, among which are the proapoptotic serine proteases (granzymes) to deliver a lethal hit to the target cell.1,2 The key role of perforin in immune surveillance has been extensively investigated using perforin knockout (PRF-KO) mice, which display a high sensitivity to several viral infections; develop spontaneous, aggressive disseminated B-cell lymphoma; and fail to optimally reject many transplanted tumors.3–6
The first description of an inherited perforin deficiency in humans was in 1999, manifested as a hemophagocytic syndrome termed type 2 familial hemophagocytic lymphohistiocytosis (FHL2).7 Most missense PRF1 mutations in FHL2 patients result in loss of function at the “presynaptic” level, most commonly due to unfolding and/or faulty trafficking of the protein.8,9 Recently, mutations in vesicular trafficking proteins such as Munc13-410 and Syntaxin 1111 have also been shown to play a causative role in the disease. Collectively, mutations affecting Munc 13-4, Syntaxin 11, and perforin are responsible for only 30% to 70% cases of the disease, depending on sanguinity, suggesting that other genetic defects leading or predisposing to FHL remain to be identified.
The rarity of FHL (estimated to be 1 in 50 000 live births for all causes and about 1 in 90 000 live births for perforin-associated disease) reflects the fact that inactivating mutations of PRF1 are very uncommon. However, a far more contentious issue in clinical immunology has been the pathophysiological role of a common polymorphism, C272T, found in up to 20% of healthy individuals (generally around 8%) in various population-based studies.8,12–14 This polymorphism leads to the conservative amino acid substitution, Ala to Val at residue 91 (A91V). With the exception of some fish species that have a similarly sized serine residue at this position,15 alanine is invariant in all other species examined to date. Several studies have suggested a causal link between A91V and atypical (late) presentation of FHL2.14,16–18 A91V has been also proposed to predispose to various types of cancer, including T- and B-cell lymphoma19 and childhood acute lymphoblastic leukemia (ALL).20 In contrast, another study has found no statistically significant difference between the frequency of A91V polymorphism in control and ALL patients but identified an increased incidence of the mutation in ALL cases harboring the bcr/abl (“Philadelphia”)12 translocation. A91V has also been linked to Dianzani lymphoproliferative disease.21 In most of these cases (except FHL), A91V was found only on one PRF1 allele, whereas the other allele was normal. Conversely, however, of 12 reported cases of A91V homozygosity, there has been 1 incident of late atypical FHL,14 1 incident of chronic lymphocytic leukemia,14 and 4 cases of ALL.12,20,21 Although most clinical observations implying a pathogenic (or a strong risk factor) role for A91V have been based on small sample sizes, they raise an important question of the role of perforin and A91V polymorphism in immune homoeostasis.
Over the past 2 years, there were several attempts to investigate the functional defects associated with A91V polymorphism.22–24 However, these studies were either based on the analysis of cytotoxic lymphocytes from an FHL patient22 or perforin expressed in rat basophil leukemia cells (RBL).23,24 Although the common finding of these studies was an apparent reduction in steady state levels of endogenous or recombinant A91V and its mouse counterpart, A90V, in effector cells, none of these studies directly addressed the specific cellular or molecular defects in A91V function. One fundamental problem with reconstituting human perforin function in vitro is that no suitable cellular system exists to perform these studies. RBL cells have proven to be an appropriate system for studying mouse perforin expression and function,25,26 but they were suboptimal for studying human homologs.27 Overall, the field lacks any evidence that moderately reduced intracellular levels of perforin would have functional significance at the level of CLs. Furthermore, the precise effect of A91V substitution on the lytic function of perforin is also currently unknown.
In the current study, we resolved these problems by designing and conducting the first perforin complementation assay using perforin-knockout primary CTLs. This novel assay allowed the assessment of perforin function in the physiological environment of the most appropriate effector cells, cytotoxic lymphocytes, where perforin is expressed in the most ideal setting to facilitate its synthesis, trafficking, and storage. In this setting, perforin also exerts its cytotoxic function in concert with granzymes rather than solely through cytolysis.1,2 We have also used a range of technologies to provide comprehensive functional analysis of the mutant protein. We showed here that A91V polymorphism results in impaired perforin function manifested both presynaptically and postsynaptically, and we discuss our findings with respect to the potential role of A91V in human disease and cytotoxic lymphocyte function in general.
Materials and methods
Cell culture, cytotoxicity assays, and perforin expression
Site-directed mutagenesis of perforin cDNA using the QuickChange methodology (Stratagene, La Jolla, CA); the culture, transient transfection, and fluorescence-activated cell sorting (FACS) of RBL-2H4 cells; the 51Cr release cytotoxicity assay; sheep red blood cell (SRBC) lysis assay; perforin expression; and purification from baculovirus-infected Sf9 cells were all performed according to the methods described by us earlier.23,26
To investigate intracellular stability of the wild-type and mutant PRF, RBL-2H4 cells were transfected with human wild-type (hPRF-WT) and A91V (hPRF-A91V) mutant DNA constructs as described elsewhere.23,26 After 16 hours of culture, the cells were treated with 2 μM cyclohexamide for up to 8 hours. The cells were recovered by trypsin treatment, and an equal number of cells was lysed as described by us earlier.23,26 Perforin was subsequently detected using Western blotting. Signal intensity was estimated using laser densitometry. The results were standardized relative to the level of perforin expression at time “0.”
To investigate a potential dominant negative effect of hPRF-A91V, a constant amount of wild-type perforin was premixed with hPRF-A91V at various ratios and added to 2 × 107 SRBCs (per reaction) in 20 mM HEPES, 150 mM NaCl, 1 mM CaCl2 buffer.23,26 The level of cell lysis, measured by the release of hemoglobin, was estimated as described earlier.23,26
Expression of perforin in mouse perforin-deficient lymphocytes
Perforin-knockout mice (PRF-KO; BL/6) were bred with BL/6 mice expressing the T-cell receptor transgene OT-1, and the F1 mice were intercrossed to enable selection of OT-1–expressing perforin-null mice. To complement perforin expression, CD8+ splenocytes were cultured in 6-well plates at a 1:10 responder-stimulator ratio with irradiated BL/6 splenocytes that had been previously pulsed at 37°C for 60 minutes with SIINFEKL peptide (presented on H-2Kb, 1 μg/mL) and thoroughly washed. The cells were cultured in RPMI-1640 medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal calf serum, 2 mM glutamine, 1 mM pyruvate, nonessential amino acids, and 2 mM mercaptoethanol for 48 hours and used in gene transfection experiments. The activated cells were analyzed for CD8, CD3, CD25, CD44, and CD69 expression using a cytofluorograph to ensure an activated phenotype had been achieved (data not shown). For perforin expression, 1 × 107 activated T cells were transiently transfected using an Amaxa electroporator and the Nucleofector mouse T-cell kit (Amaxa Biosystems, Cologne, Germany) with wild-type or mutated perforin cDNA that had been cloned into the pIRES2-EGFP plasmid (Clontech, Mountain View, CA). About 20 to 24 hours later, cells expressing equivalent levels of EGFP were sorted and isolated in the same fashion as we described for RBL-2H4 cells.23,26 The resultant GFP-positive T cells were used in a 4-hour 51Cr release assay against 51Cr-labeled EL-4 thymoma cell targets pulsed with 1 mg/mL SIINFEKL peptide for 1 hour in serumfree culture media or EG7.ova (a derivative of EL-4; H-2b).
Gel filtration chromatography
The gel filtration of purified, recombinant native perforin or perforin reduced by incubation in buffer containing 10 mM dithiothreitol (DTT) for 5 minutes was performed by high-performance liquid chromatography (HPLC) on a Superdex-200 column (GE Healthcare, Uppsala, Sweden) equilibrated with 270 mM imidazole per 300 mM NaCl (pH 8.0) (perforin elution buffer). The column was calibrated using the molecular-weight standards thyroglobulin (669 kDa; eluted in fractions 1 to 6), catalase (232 kDa; eluted in fractions 7 to 10), and bovine serum albumin (67 kDa; eluted in fractions 11 to 13).
Western blotting
Cell lysates or purified recombinant perforin were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, transferred to PVDF membranes, and probed for perforin or actin expression using the rat antiperforin monoclonal antibody (mAb) P1-8 and the mouse antiactin mAb and appropriate HRP-conjugated Ig as reported earlier.23
Results
The common human perforin polymorphism A91V has attracted a great deal of interest over the last 2 to 3 years, as several associative studies suggested that the mutation might predispose to immune deficiency disorders. In the current study, we conducted the first comprehensive analysis of hPRF-A91V function at the cellular and molecular levels and demonstrated that the mutant protein malfunctions both at presynaptic and postsynaptic levels.
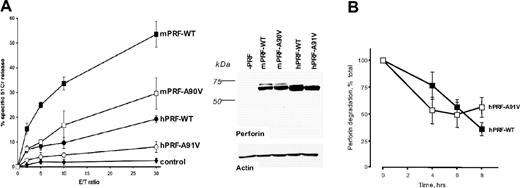
A fundamental problem with reconstituting human perforin function is that no cell lines are ideally suited for this purpose. Risma et al previously demonstrated the aberrant migration in nonreducing SDS-PAGE of A91V perforin expressed in rat RBL-1 cells. However, these cells are unsuitable for assessing cytotoxicity, because they are not able to efficiently exocytose their granule contents.24 In attempting to address these issues, we transiently transfected RBL-2H4 cells (which are capable of efficient exocytosis) with DNA constructs encoding hPRF-WT and hPRF-A91V and compared their capacity to kill trinitrophenol (TNP) surface-labeled Jurkat target cells to which they were conjugated through their Fcϵ receptors and anti-TNP IgE.25 As detected by Western blotting, both forms of human perforin were expressed slightly more abundantly than wild-type mouse perforin that was used as a positive control for expression and function (see Western blot in Figure 1A). Mouse perforin (mPRF-WT) induced strong cell lysis (30% to 35% specific 51Cr release at an E/T ratio of 10), whereas cytotoxicity with mPRF-A90V, the mouse equivalent of hPRF-A91V, was substantially reduced (Figure 1A), consistent with our earlier report.23 However, the level of cytotoxicity that could be achieved with hPRF-WT was much lower than with mouse perforin, even at an E/T ratio of 30 (Figure 1A). By comparison, hPRF-A91V induced yet lower target cell lysis that was only slightly greater than the GFP-expressing vector backbone alone (Figure 1). Even though the hPRF-A91V mutant protein was consistently expressed at somewhat lower levels than hPRF-WT, there was no detectable difference between their half-life in RBL cells (approximately 8 hours) (Figure 1B).
Comparative analysis of mouse and human perforin in RBL cells. (A) The experiment shown is a representative 51Cr release assay of RBL cells transfected with wild-type murine (mPRF-WT) or human (hPRF-WT) perforin or homologous mutants, mPRF-A90V or hPRF-A91V (mean ± SD). The Western blot shows relative levels of expression of human and mouse perforin in RBL cells. (B) Relative half-lives of the wild-type and A91V mutant perforin expressed in RBL cells. Shown is mean ± SE of 3 independent experiments. Intracellular levels of perforin at time “0” (prior to adding cyclohexamide) were designated as 100%.
Comparative analysis of mouse and human perforin in RBL cells. (A) The experiment shown is a representative 51Cr release assay of RBL cells transfected with wild-type murine (mPRF-WT) or human (hPRF-WT) perforin or homologous mutants, mPRF-A90V or hPRF-A91V (mean ± SD). The Western blot shows relative levels of expression of human and mouse perforin in RBL cells. (B) Relative half-lives of the wild-type and A91V mutant perforin expressed in RBL cells. Shown is mean ± SE of 3 independent experiments. Intracellular levels of perforin at time “0” (prior to adding cyclohexamide) were designated as 100%.
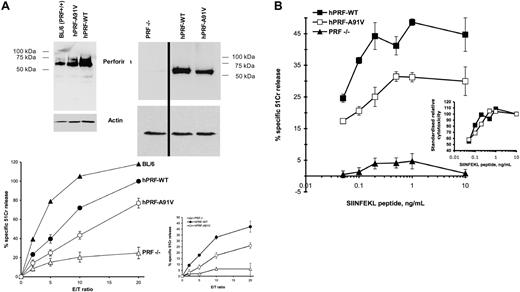
As the posttranslational instability of hPRF-A91V in RBL-2H4 cells coincided with a substantial reduction in cytotoxic activity compared with hPRF-WT, we wished to analyze human perforin in a more physiological setting and one in which killing was more optimized than RBL-2H4 cells. We therefore opted to reconstitute perforin function in the primary CTLs of perforin gene knockout mice. To examine cytotoxic function in an antigen- and perforin-dependent setting, we performed our experiments in CTLs of perforin-knockout BL/6 mice that also express a transgenic T-cell receptor that enables recognition of the ovalbumin peptide SIINFEKL on cells such as EL-4 thymoma cells (H-2Kb). Western immunoblots confirmed that both hPRF-WT and hPRF-A91V were expressed in the CD8+ perforin-deficient lymphocytes, although the steady state expression levels for A91V were consistently lower than for wild-type perforin (Figure 2A), similar to our findings in RBL-2H4 cells (Figure 1 and Voskoboinik et al23 ). By comparing the relative numbers of T cells required to induce a given level of cell death, we found that hPRF-A91V-expressing T cells induced approximately half the level of 51Cr release from EL-4 cells as wild-type perforin (Figure 2A). Target cell death was perforin dependent, as perforin-null OT-1 T cells produced only background levels of cell death, whereas the CTLs of BL/6 control mice induced near maximal cell death with an effector-target (E/T) ratio of 10 (Figure 2A). Similarly, perforin-null CTLs transfected with an inactive mutant of perforin,28 D484A, had no cytolytic activity (our unpublished observations, December 2006).
Complementation of the PRF-KO phenotype of primary mouse CTLs. Primary mouse CTLs were generated, activated, transfected to express hPRF-WT or hPRF-A91V perforin, sorted, and tested in cytotoxicity assay against SIINFEKL-pulsed EL-4 target cells as described in “Materials and methods.” (A) Data in the larger graph represent the mean ± SE of 4 to 6 independent experiments, and 2 typical Western immunoblots are shown. Each independent experiment was standardized with respect to the level of 51Cr release induced by hPRF-WT-transfected cells in 20:1 E/T ratio (100%). Activated CTLs from BL/6 mice were used as control. The smaller graph shows the outcome of one typical experiment (mean ± SD of triplicate data points; raw data are shown). The effect of antigen presentation was tested by treating 51Cr-labeled EL-4 target cells with various concentrations of SIINFEKL peptide. The effector cells were used in 15:1 E/T ratio. (B) Data from a representative experiment are shown as mean ± SD. Inset shows standardized data from a large graph, where the level of specific 51Cr release from EL-4 targets treated with 10 ng/mL peptide was regarded as 100%.
Complementation of the PRF-KO phenotype of primary mouse CTLs. Primary mouse CTLs were generated, activated, transfected to express hPRF-WT or hPRF-A91V perforin, sorted, and tested in cytotoxicity assay against SIINFEKL-pulsed EL-4 target cells as described in “Materials and methods.” (A) Data in the larger graph represent the mean ± SE of 4 to 6 independent experiments, and 2 typical Western immunoblots are shown. Each independent experiment was standardized with respect to the level of 51Cr release induced by hPRF-WT-transfected cells in 20:1 E/T ratio (100%). Activated CTLs from BL/6 mice were used as control. The smaller graph shows the outcome of one typical experiment (mean ± SD of triplicate data points; raw data are shown). The effect of antigen presentation was tested by treating 51Cr-labeled EL-4 target cells with various concentrations of SIINFEKL peptide. The effector cells were used in 15:1 E/T ratio. (B) Data from a representative experiment are shown as mean ± SD. Inset shows standardized data from a large graph, where the level of specific 51Cr release from EL-4 targets treated with 10 ng/mL peptide was regarded as 100%.
To confirm that the difference between cytotoxic activities of the hPRF-WT- and the hPRF-A91V-expressing CTLs was independent of the level of antigen presentation but was a function of perforin activity, we conducted the killing assay when the E/T ratio remained constant (15:1) and various concentrations of SIINFEKL were used to pulse the target cells (Figure 2B). This approach provided conditions of limited antigen presentation. As expected, the decay of cytotoxicity as a function of decreasing SIINFEKL concentrations was not different whether the wild-type or A91V perforin were expressed (Figure 2B, inset).
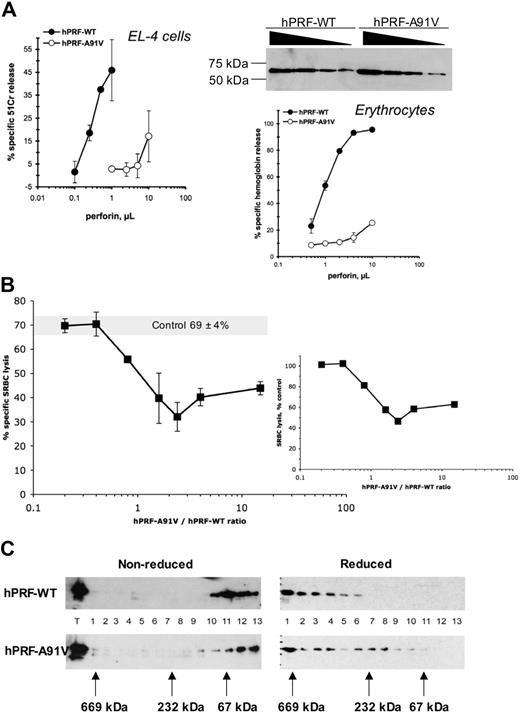
Because hPRF-A91V was less cytotoxic than hPRF-WT when expressed in both RBL-2H4 cells and primary lymphocytes, it was important to identify a possible cause(s) for this. As hPRF-A91V expression levels were consistently lower in both models, one possibility was that reduced target cell death simply reflected the fact that less perforin was delivered to the target cells upon CTL degranulation. The second possibility was that A91V also resulted in an intrinsic reduction in lytic activity. To distinguish these possibilities, we used the baculovirus expression system to purify hPRF-A91V and hPRF-WT and directly compared their lytic activity. Remarkably, we found that the A91V mutant had a massively (at least 10-fold) reduced cytolytic activity on both nucleated (EL-4) and nonnucleated (erythrocytes) cells compared with an equal amount of wild-type protein (Figure 3A).
Cytolytic activity of purified recombinant perforin. (A) Human wild-type and A91V mutant perforins were purified using a baculovirus system; their respective concentrations were equilibrated using Western blots and the cytolytic activity tested using SRBC lysis assay or 51Cr release from EL-4 cells (mean ± SD). (B) Purified recombinant hPRF-A91V and hPRF-WT proteins were mixed at 0:1, 0.2:1, 0.4:1, 0.8:1, 1.6:1, 2.4:1, 4:1, and 12:1 ratios and then added to SRBCs. The large graph shows nonstandardised (raw) levels of SRBC lysis in which 69% ± 4% lysis corresponds to the addition of hPRF-WT alone to the cells. The data are shown as mean ± SE of 3 to 4 independent experiments and performed with 2 independent batches of perforin. The small graph depicts standardized values shown on the large graph, where “control” was designated as 100%. (C) Purified recombinant WT and A91V perforins were subjected to the gel filtration, and the elution profile was analyzed using Western blots (see “Western blotting” in “Materials and methods” for details).
Cytolytic activity of purified recombinant perforin. (A) Human wild-type and A91V mutant perforins were purified using a baculovirus system; their respective concentrations were equilibrated using Western blots and the cytolytic activity tested using SRBC lysis assay or 51Cr release from EL-4 cells (mean ± SD). (B) Purified recombinant hPRF-A91V and hPRF-WT proteins were mixed at 0:1, 0.2:1, 0.4:1, 0.8:1, 1.6:1, 2.4:1, 4:1, and 12:1 ratios and then added to SRBCs. The large graph shows nonstandardised (raw) levels of SRBC lysis in which 69% ± 4% lysis corresponds to the addition of hPRF-WT alone to the cells. The data are shown as mean ± SE of 3 to 4 independent experiments and performed with 2 independent batches of perforin. The small graph depicts standardized values shown on the large graph, where “control” was designated as 100%. (C) Purified recombinant WT and A91V perforins were subjected to the gel filtration, and the elution profile was analyzed using Western blots (see “Western blotting” in “Materials and methods” for details).
Given the ability of hPRF-A91V to complement the function of PRF-KO CTLs (Figure 2A), it was clear that the membrane-binding and pore-forming properties of the mutant were not totally abolished. Because it was not possible to coexpress equal amounts of hPRF-WT and hPRF-A91V in primary CTLs to mimic the heterozygous state, we tested hPRF-A91V for a potential dominant negative effect on hPRF-WT using purified recombinant proteins. To test this, the proteins were mixed at various hPRF-A91V/hPRF-WT ratios and then added to target cells. The results indicated that at protein ratios 0.2:1 and 0.4:1 there was no detectable effect of the mutant perforin on the activity of its wild-type counterpart. A marginal (20%) inhibitory effect was found with a 0.8:1 ratio of proteins, and a significant 40% to 50% decrease in the cytolytic function of hPRF-WT was observed only with a 1.6- to 4-fold excess of hPRF-A91V (Figure 3B).
Given the relatively conserved nature of the alanine for valine (which differ by just 2 methyl groups) substitution, a large difference between the cytolytic activities of hPRF-WT and hPRF-A91V was surprising. We therefore hypothesised that such an effect could be at least partly attributed to abnormal folding of the mutant protein, which could also be responsible for its reduced steady state expression levels (Figures 1,2). Perforin is tightly folded through 10 disulfide bridges. Therefore, one of the outcomes of the mutation could be an effect on the formation of intermolecular complexes. To address this issue, we compared the aggregation of purified recombinant hPRF-WT and hPRF-A91V proteins using gel filtration chromatography. Whereas under nonreducing conditions both perforins were largely found in fractions 10 to 13 (corresponding in size to monomeric perforin), exposure to reducing conditions with DTT resulted in the formation of high molecular weight complexes of more than 232 kDa (corresponding to aggregates composed of at least 4 monomers), as indicated by perforin elution in column fractions 1 to 6 (Figure 3C). However, unlike hPRF-WT, the mutant was additionally found in fractions 7 to 11, indicating the added presence of monomers, dimmers, and trimers (Figure 3B). Overall, our results suggest the A91V mutation had a structural effect on perforin, manifested as an impaired capacity to spontaneously aggregate under reducing conditions.
Taken together, the outcome of these experiments clearly showed that the A91V substitution in perforin not only causes reduced steady state levels of expression in effector cells (presynaptic dysfunction) but also results in a reduced intrinsic capacity for lysis and even had some dominant negative effect that can be demonstrated with purified perforin applied directly to target cells (postsynaptic dysfunction).
Discussion
Several reports have recently linked the A91V polymorphism of perforin to a variety of pathological conditions including lymphoma, acute childhood lymphocytic leukemia, and Dianzani syndrome12,19–21 as well as atypical (late-onset) FHL.14,16–18 However, the high frequency of healthy A91V carriers in the general population (approximately 20%) is far more in keeping with a neutral polymorphism. To resolve this apparent paradox, we applied a range of functional and physical assays to ascertain the molecular and functional properties of hPRF-A91V. In all 3 experimental systems we examined (primary CTLs, RBL-2H4 cells, and purified protein), the level of hPRF-A91V expression was reduced compared with hPRF-WT. We have also made similar observations with the mouse perforin A90V mutant homolog using these same expression systems23 (also our unpublished observations, August and September 2005, February 2006).
Given the limitations associated with the use of a basophil cell line as a surrogate CTL for studying human perforin, we opted to express perforin in CD8+ mouse CTLs in which the perforin gene had been structurally disrupted. By crossing these mice with syngeneic mice overexpressing a clonal T-cell receptor (OT-1), we were able to reconstitute perforin-dependent cytotoxic function in an antigen-dependent manner. We demonstrated for the first time that human perforin could restore the cytotoxic activity of PRF-KO lymphocytes but that hPRF-A91V was only approximately half as efficient in clearing the same ovalbumin-expressing target cells. Taking into account that hPRF-A91V was expressed at slightly lower levels in these cells than hPRF-WT, we initially hypothesized that this finding alone might be sufficient to explain the moderate reduction in the cytotoxic function of the mutant perforin-transfected CTLs. However, that did not appear to be the case in the context of mouse perforin homologs, where a substantial reduction of mPRF-A90V expression compared with mPRF-WT did not translate into any significant effect in CTL cytotoxicity (our unpublished observations, August and September 2005, February 2006). It was therefore possible that the hPRF-A91V mutation could result in perforin with intrinsically reduced cytotoxic activity. Indeed, we found the mutant protein was at least 10-fold less active than hPRF-WT in inducing lysis when applied directly to target cells (Figure 3A). Furthermore, purified recombinant hPRF-A91V displayed a mild dominant negative effect but only when added at a 1.6- to 4-fold excess, a range of ratios that is very unlikely to occur in intact CTLs due to A91V's inherent instability (Figures 1A and 2A). The dominant negative effect was not significant at 0.4:1 and was only marginal at 0.8:1 mutant-wild-type ratios (Figure 3B), scenarios we have shown experimentally to be more likely A91V/WT homozygous individuals.
One of the most exciting yet unexpected outcomes of the study was the observation that the major intrinsic dysfunction of hPRF-A91V results in a relatively mild impact on the function of primary CTLs. The most likely explanation is that the diminished lytic capacity of hPRF-A91V might be partly compensated in CTLs by its codelivery with other granule toxins, particularly the granzymes. If this is the case, our study demonstrates for the first time that even a significant impairment in perforin function (as observed here with hPRF-A91V) might still provide sufficient activity to maintain normal immune homeostasis in vivo.9 Consistent with this hypothesis, it has been recognized that the constitutive level of NK cytotoxicity can vary considerably (up to 10-fold) among healthy individuals. A spectrum of intrinsic perforin activity and/or levels of expression may be one of a number of factors contributing to the normal variation in cytotoxic capacity. Even under pathological conditions, such as FHL, the age at onset of the disease may be delayed considerably due to the residual activity of perforin in CTL/NK cells.
The fact that a common allele such as A91V results in a significant reduction in perforin cytotoxicity invites the following question: What is the minimal level of perforin activity required to maintain immune homeostasis and avoid disease? Clearly, the single normal perforin allele expressed by parents of children with FHL is sufficient to maintain health and, assuming codominant allelic expression, such individuals would be expected to have 50% of normal perforin activity. Similarly, normal immune homoeostasis would be expected in a person with 1 normal and 1 A91V allele (no dominant negative effect of A91V on wild-type perforin activity was observed under “physiologically” relevant conditions, as discussed in the second paragraph of this section and shown in Figure 3B). If we assume that A91V results in about 50% loss of CTL activity, an A91V homozygous individual would also maintain about 50% normal perforin function and might generally be expected to remain healthy. However, there may be a difference between the 50% activity provided by a single allele of wild-type perforin and 2 alleles of A91V, which was shown here to have significantly reduced cytolytic activity per se. Indeed, of 12 reported cases of A91V homozygosity, 7 have developed either a late form of IHL14 or leukemia12,14,20 or Dianzani autoimmune lymphoproliferative disorder.21 Other individuals were asymptomatic at the time of the study, but at least one has been reported as having “extremely reduced” perforin level in CTLs.14 Taken together, these data suggested that A91V homozygosity might represent a strong risk factor for disease. At the same time, inheriting hPRF-A91V in conjunction with another mutated PRF1 allele encoding a “null” mutation would be expected to result in a further substantial reduction in perforin function (to no more than 25% of normal perforin) that would most certainly compromise immune homeostasis, so this genotype would be a disease-causing one. Indeed, several such patients with late-onset FHL16–18 and one case of lymphoma have been reported.19
Although we identified no pronounced dominant negative effect, this might become relevant if the wild-type allele were expressed at abnormally low levels. A recent study has shown that transcriptional regulation of the PRF1 gene is controlled by a 150 kb-long active chromatin domain.29 Polymorphisms in this region have never been investigated in relation to the level of perforin expression under normal or pathological conditions. We postulate that the observed variations in the levels of perforin expression and CTL/NK cell cytotoxicity in healthy populations may partly be due to differences in the efficiency of PRF1 transcription. Should the level of a structurally normal perforin protein be reduced in relation to hPRF-A91V through such a mechanism, it is conceivable that a relative excess of the mutant protein might reduce the lytic function of hPRF-WT with a negative effect on overall cytotoxicity. Such a dominant negative effect might be far more likely if amplified if hPRF-A91V were inherited with an allele that encodes perforin protein that has less than WT activity. Indeed, while still being somewhat controversial, there are reports of the link between A91V heterozygocity and various types of immunogenic cancers.19
How can such a high frequency of potentially disease-causing A91V mutation be explained? One reason might be that the decreased function of perforin and, consequently, cytotoxic lymphocytes could protect an individual from an autoimmune disease should it be common within a particular population. There is as yet no evidence supporting this hypothesis, although A91V has been reported as a low-frequency allele in certain populations, such as African Americans in the United States.12 Another possible explanation is that manifestation of perforin deficiency due to A91V, even in extreme cases of atypical FHL, is usually delayed to at least the late teenage years. Consequently, the selective pressure against A91V may be weak, because disease may not occur before reproductive age. This is in contrast to the strongly positive selective pressure on certain genetic polymorphisms that confer resistance to malaria, a disease that causes particularly high mortality among infants and pregnant women. Among such gene products are hemoglobin, glucose-6-phosphate dehydrogenase, and interleukin-4.30 A degree of resistance to a very recent infectious agent, HIV-1, as a result of a mutated HIV-1 coreceptor CCR5-Δ32 appears to have been linked to protection against bubonic plague and smallpox in the more distant past. In this case, the frequency of the mutant allele appears to have increased significantly over the past few centuries, leading to somewhat reduced mortality from both diseases.31
In conclusion, we conducted functional characterization of a common perforin polymorphism, A91V, using a novel experimental system in which we have complemented the cytotoxic function of perforin-null CTLs. We found that the A91V mutation is far more detrimental for perforin function than was initially anticipated, because it affects perforin stability within the effector cell and greatly reduces its intrinsic cytolytic activity. These deficiencies, however, can be partially “rescued” by the physiological environment of a cytotoxic lymphocyte, where even a suboptimal level of perforin activity may still be sufficient for synergy with proapoptotic components of secretory granules to destroy the target cell. Our findings are consistent with several epidemiologic reports showing a high frequency of A91V heterozygotes in the general population. On the basis of those studies and our experimental evidence, we propose that the A91V polymorphism can predispose to disease associated with partial immune deficiency if inherited as a homozygote and may particularly be disease-causing if it is inherited with a second perforin allele with “null” activity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a Project Grant and the R. D. Wright Fellowship from the National Health and Medical Research Council (NHMRC) of Australia (I.V.) and Senior Fellowship and Program Grants from the Juvenile Diabetes Research Foundation and from the NHMRC of Australia (J.A.T.).
Authorship
Contribution: I.V. designed and performed research and drafted the manuscript; V.R.S. designed and performed research; A.C. and J.C. performed research and collected data; C.H. contributed analytical tools and interpreted data; P.K.D. and H.Y. contributed vital new reagents and analytical tools; and J.A.T. designed research, analyzed and interpreted data, and helped draft the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ilia Voskoboinik; e-mail: ilia.voskoboinik@petermac.org; or Joseph A. Trapani; e-mail: joe.trapani@petermac.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal