Abstract
Activated coagulation factor XIII (FXIIIa) cross-links the γ-chains of fibrin early in clot formation. Cross-linking of the α-chains occurs more slowly, leading to high molecular weight multimer formations that can also contain γ-chains. To study the contribution of FXIIIa-induced γ-chain cross-linking on fibrin structure and function, we created 2 recombinant fibrinogens (γQ398N/Q399N/K406R and γK406R) that modify the γ-chain cross-linking process. In γK406R, γ-dimer cross-links were absent, but FXIIIa produced a cross-linking pattern similar to that observed in tissue transglutaminase cross-linked fibrin(ogen) with mainly α-γ cross-links. In Q398N/Q399N/K406R, cross-links with any γ-chain involvement were completely absent, and only α-chain cross-linking occurred. Upon cross-linking, recombinant normal fibrin yielded a 3.5-fold increase in stiffness, compared with a 2.5-fold increase by α-chain cross-linking alone (γQ398N/Q399N/K406R). γK406R fibrin showed a 1.5-fold increase in stiffness after cross-linking. No major differences in clot morphology, polymerization, and lysis rates were observed, although fiber diameter was slightly lower in cross-linked normal fibrin relative to the variants. Our results show that γ-chain cross-linking contributes significantly to clot stiffness, in particular through γ-dimer formation; α-γ hybrid cross-links had the smallest impact on clot stiffness.
Introduction
After cross-linking by FXIIIa, fibrin becomes markedly more resistant to proteolytic and mechanical disruption. The introduction of Nϵ-(γ-glutamyl)lysine isopeptide bonds between fibrin molecules within and between clot fibers has a remarkable effect on the rheologic properties of clots.1-4 This stabilization of fibrin fibers leads to the formation of a rigid and elastic structure that is capable of stopping leakages in the circulatory system, whereas bleeding is a frequent problem without FXIIIa cross-linking. Of the 3 fibrinogen chains, only the α- and γ-chains, but not the β-chains, become cross-linked by FXIIIa. During clot formation, at the early stages of polymerization, cross-linking occurs within emerging protofibrils between γK406 and γQ398 and/or γQ399, resulting in the formation of γ-dimers.5-7 Multiple cross-linking between fibrin α-chains results in the formation of α-polymers.6 Over time, FXIIIa has been shown to generate cross-linked chain structures containing various combinations of α- and/or γ-chains; mainly these are thought to be αn,8 but γ3, γ4, and hybrid αpγq (n, p, and q = 1, 2, 3, respectively, and so forth) are also known to occur.9,10 The effect of these different cross-linking formations on fibrin clot function has not been fully clarified. Previously, the increase of stiffness in clots has been attributed to α-chain cross-linking.11 On the other hand, a recent study suggested that γ-chain cross-linking played a major role in the determination of the viscoelastic properties of fibrin.12 However, in these studies, chain-specific cross-linking was manipulated either by antibodies or by the use of truncated molecules, with inherent difficulties in interpretation arising from the effects of these modifications themselves on fibrin structure and function. We chose a different approach by creating full-length fibrinogen variants with substitutions in the FXIII cross-linking sites on the fibrinogen γ-chain to enable us to study the contribution of γ-chain cross-linking to fibrin structure and function. In the experiments reported here, we examined the structural and physical properties of clots using these variants with modified γ-chain cross-linking
Materials and methods
Vector construction and expression of recombinant fibrinogens
The expression vector pMLP-γ, which contains the entire fibrinogen γ-chain cDNA, has been described elsewhere.13 To create γK406R, codon 406 was altered from AAA (lysine) to CGA (arginine) using the QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). The following primers (Invitrogen, Poole, United Kingdom) were used: 5′-CACCACCTGGGGGGAGCCCGACAGGCTGGAGACGTTTAAAAG-3′ and 5′-CTTTTAAACGTCTCCAGCCTGTCGGGCTCCCCCCAGGTGGTG-3′ (mutated codon in bold). The resulting vector was used as a template to create γQ398N/Q399N/K406R with the following primers: 5′-CTCACAATTGGAGAAGGAAACAACCACCACCTGGGGGG-3′ and 5′-CCCCCCAGGTGGTGGTTGTTTCCTTCTCCAATTGTGA-3′ (mutated codons in bold). Mutations were confirmed by sequencing using an ABI PRISM312 automated sequencer (Applied Biosystems, Warrington, United Kingdom). Chinese hamster ovary cells expressing the human Aα and Bβ fibrinogen chains were cotransfected with the variant γ-chain vectors and a plasmid containing a selection gene, pMSV his.13 Normal and variant fibrinogen were expressed and purified as previously described.14 Purified proteins were dialyzed against 100 mmol l−1 NaCl, 50 mmol l−1 Tris, pH 7.4, and stored at −80°C. The same buffer was used throughout the entire study for the formation of fibrin clots. Purity of preparations was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing and nonreducing conditions following the method of Laemmli.15
Preparation of FXIII
A plasma-derived concentrate of FXIII, used clinically for the treatment of patients with FXIII deficiency (fibrogammin P; ZLBBehring, Sussex, United Kingdom), was separated from contaminating albumin and glucose using gel filtration. In brief, 250 U fibrogammin dissolved in 20 mmol l−1 Hepes, pH 7.4, was separated on Sepharose 6B (2.6 × 38-cm column) using a BioCad Sprint Chromatography system (Applied Biosystems), with 20 mmol l−1 Hepes, pH 7.4, and a flow rate of 0.5 mL min−1. Fractions containing FXIII A2B2 tetramers were concentrated by centrifugation using Amicon Centriplus YM-100 centrifugal devices (Millipore, Billerica, MA), dialyzed against 100 mmol l−1 NaCl, 50 mmol l−1 Tris, pH 7.4, and stored at −80°C. The FXIII obtained by this procedure was pure as determined by SDS-PAGE and of similar activity as plasma-purified FXIII used in our previous studies.16
Cross-linking of fibrin by FXIIIa
Fibrinogen (0.4 mg mL−1 final concentration) and 0.022 mg mL−1 FXIII in 100 mmol l−1 NaCl, 50 mmol l−1 Tris, pH 7.4, were incubated with 0.25 U mL−1 human α-thrombin (Calbiochem, Darmstadt, Germany) and 5 mmol l−1 CaCl2 for 0, 5,10, 30, 60, and 180 minutes at 37°C. The reaction was stopped by the addition of reducing sample buffer (Invitrogen) and heating the samples for 5 minutes at 85°C. Samples were separated on a 3% to 8% Tris-acetate gradient gel (Invitrogen). Proteins were visualized on the gels using GelCode blue stain reagent (Pierce, Rockport, IL).
Cross-linking of fibrin by guinea pig liver transglutaminase
Fibrinogen (0.4 mg mL−1) was cross-linked upon addition of 1 U mL−1 guinea pig tissue liver transglutaminase (tTG; Sigma) and 10 mmol l−1 CaCl2 and 0.25 U mL−1 human α-thrombin. Samples were incubated at 37°C for 180 minutes and processed as described under “Cross-linking of fibrin by FXIIIa.”
Identification of cross-linking products
The composition of cross-linking products was determined by immunoblotting and matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). Samples were incubated at 37°C for one hour, separated by SDS-PAGE as described above, and transferred to a nitrocellulose membrane (Pierce). To distinguish between α- and γ-chain formations, 2 murine monoclonal antibodies were used, directed against residues 15-35 in the γ-chain and 290-348/349-406 in the α-chain (both from Accurate Chemical & Scientific, Westbury, NJ); a peroxidase-conjugated secondary antibody was used for detection with tetramethyl benzidine (Calbiochem). All bands of interest were excised from the gel after SDS-PAGE, digested with trypsin, and analyzed by MALDI-MS.
Viscoelastic properties of fibrin clots
Clots were prepared between 2 12-mm-diameter glass coverslips that were positioned 1 mm apart in a torsion pendulum as previously described.17 Recombinant fibrinogen variants and plasma purified fibrinogen (Calbiochem; further purified by IF-1 affinity chromatography18 ) were incubated at 0.75 mg mL−1 with 10 mmol l−1 CaCl2 and 1 U mL−1 human α-thrombin, with or without 0.022 mg mL−1 FXIII. Sixty minutes after initiation of clotting, a momentary impulse was applied to one arm of the torsion pendulum by air pressure, which caused free oscillations of the arm with strains of less than 3%. The oscillations were recorded on a chart recorder and used to calculate the storage modulus, G′, in dyne cm−2, which reflects the stiffness of the fibrin clot.17
Polymerization of fibrinogen and macroscopic fibrinolysis velocity by turbidity
Polymerization of normal and variant recombinant fibrinogens (final concentration 0.33 mg mL−1) was measured in a microtiter plate turbidity assay as previously described.12 Samples were incubated with 0.25 U mL−1 thrombin and 5 mmol l−1 calcium chloride in 100 mmol l−1 NaCl, 50 mmol l−1 Tris, pH 7.4, in the presence and absence of 0.022 mg mL−1 FXIII. Increase of turbidity was continually monitored every 12 seconds on a FLX-800 multiwell plate reader (Biotek, Instruments, Winooski, VT) over a period of 60 minutes at ambient temperature. After completion of polymerization at 60 minutes, all wells were overlaid with 100 μL of a mixture of 10 nmol l−1 tissue plasminogen activator (Technoclone, Vienna, Austria) and 0.05 mg mL−1 Glu-plasminogen (Calbiochem). Changes in turbidity were monitored every 12 seconds for 60 minutes, as above. After completion of lysis, cross-linked samples were reduced by the addition of SDS sample buffer and analyzed on a 3% to 8% gradient gel.
Scanning electron microscopy
Normal and variant fibrinogens at 0.4 mg mL−1 were incubated with 0.25 U mL−1 human α-thrombin and 5 mmol l−1 CaCl2, with or without 0.022 mg mL−1 FXIII. Clots were prepared for microscopy as described previously.17 Samples were observed and photographed digitally using a Field Emission Scanning Electron Microscope (LEO1530 FEGSEM; Leo Electron Microscopy, Cambridge, United Kingdom) at a magnification of 10 000 ×. Average fiber diameters of 200 fibers were measured from 5 pictures per clot by an operator blind to variant type using ImageJ 1.29× software (National Institutes of Health, Bethesda, MD).
Statistical analysis
Data were described as mean and standard deviation. Distribution of fiber diameters was normal as tested according to Kolmogorov-Smirnov. Differences in fiber diameters were investigated by analysis of variance (ANOVA) with posthoc Bonferroni analysis. All statistical analyses were performed using SPSS for Windows version 12 software (SPSS, Chicago, IL). P values lower than .05 were considered to indicate statistical significance.
Results
Cross-linking of fibrin by FXIIIa
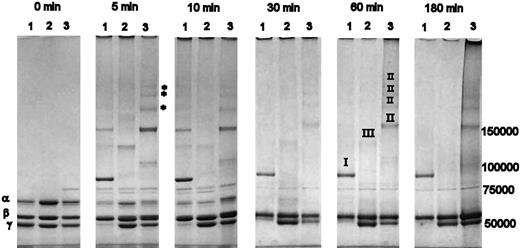
A time course of cross-linking of variant and normal fibrin by FXIIIa, resolved on a 3-8% Tris-acetate SDS-PAGE, is shown in Figure 1. After 5 minutes of cross-linking, a strong band representing γ-dimer formation was observed in normal fibrin (Figure 1 lane 1), which was absent from both γQ398N/Q399N/K406R (lane 2) and γK406R (lane 3) variants. With normal fibrin, the band for γ-chain monomer was virtually absent after 5 minutes. There was also a visible decrease of the α-band when compared with the β-band, which, due to a lack in cross-linking sites, retained the same intensity throughout the entire time course. In γK406R, a visible proportion of the α-chain was cross-linked after 5 minutes, and there was a noticeable decrease in the γ-chain band (Figure 1). Five distinct higher molecular weight bands were visible at 5 minutes, at least 3 of which (marked with * in Figure 1) appeared only in γK406R and not in the other variant or in normal cross-linked fibrin. A reduced rate of α-chain consumption during initial time points, and no involvement of the γ-chains over 180 minutes, occurred in γQ398N/Q399N/K406R (Figure 1). The cross-linking pattern of all variants changed over the first 30 minutes, with a reduction and disappearance of some bands, due to incorporation of these cross-linking species in higher molecular weight complexes that accumulated on top of the loading wells. α-Chain cross-linking appeared complete after 30 minutes in normal and γK406 and after 60 minutes in γQ398N/Q399N/K406R (Figure 1). γ-Chain cross-linking in normal fibrin was complete after 5-minute incubation, whereas cross-linking of the γ-chains in γK406R did not proceed to completion during the 3-hour incubation period, despite the early reduction in γ-monomer band intensity of this variant. There was no reduction of the γ-monomer band over 180 minutes in γQ398N/Q399N/K406R (Figure 1).
Cross-linking of fibrin variants by FXIIIa. Lane 1 of each panel: recombinant normal fibrin; lane 2: γQ398N/Q399N/K406R; lane 3: γK406R. Roman numerals in the 60-minute time point denote origin of cross-link formations as identified by MALDI-MS and Western blotting with chain-specific antibodies; I: γ-dimers, II: α-γ hybrid formation, III: α-α cross-links. After 5 minutes, nearly all γ-chains and a large proportion of the α-chain were cross-linked in normal fibrinogen. In γK406R, FXIIIa preferentially cross-linked the α-chain; after 180 minutes, a substantial amount of γ-chain remained. No γ-chain cross-linking occurred in γQ398N/Q399N/K406R. In all fibrinogens, early, relatively lower molecular weight, cross-linking bands disappeared over time, as these transient oligomers become incorporated into larger-molecular-weight molecular complexes that do not enter the gel.
Cross-linking of fibrin variants by FXIIIa. Lane 1 of each panel: recombinant normal fibrin; lane 2: γQ398N/Q399N/K406R; lane 3: γK406R. Roman numerals in the 60-minute time point denote origin of cross-link formations as identified by MALDI-MS and Western blotting with chain-specific antibodies; I: γ-dimers, II: α-γ hybrid formation, III: α-α cross-links. After 5 minutes, nearly all γ-chains and a large proportion of the α-chain were cross-linked in normal fibrinogen. In γK406R, FXIIIa preferentially cross-linked the α-chain; after 180 minutes, a substantial amount of γ-chain remained. No γ-chain cross-linking occurred in γQ398N/Q399N/K406R. In all fibrinogens, early, relatively lower molecular weight, cross-linking bands disappeared over time, as these transient oligomers become incorporated into larger-molecular-weight molecular complexes that do not enter the gel.
Identification of FXIIIa cross-linking products by immunoblotting and MALDI-MS
Immunoblotting with chain-specific antibodies and MALDI-MS analysis was used to identify the fibrin chains in the cross-linked products at 60 minutes (data not shown); the fibrinogen chains from which bands originated are designated by roman numerals in Figure 1. It was found that all high molecular weight formations in γK406R contained both α- and γ-chains, therefore representing α-γ cross-links. No distinct bands for α-α cross-linking products could be detected in γK406R, although the existence of homogenous α-α multimers that were prevented from entering the gel cannot be excluded. In normal fibrin, as expected, the γ-dimer band contained only γ-chains (Figure 1). The cross-linking products in γQ398N/Q399N/K406R did not react with the anti-γ antibody and no γ-chain signal was detected by MALDI-MS.
Cross-linking of fibrinogen and fibrin by tTG
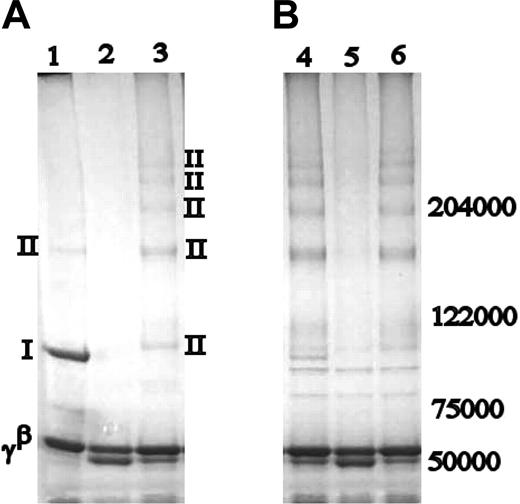
Both human red cell and guinea pig liver tTG have been shown to preferentially produce α-γ cross-link formations, at the expense of dimeric γ-chain cross-linking.19,20 It was of interest to detect whether in the absence of K406, FXIIIa would alter its cross-linking pattern of the fibrinogen γ-chain that is normally centered around γ-dimer band, leading to cross-linking products similar to those induced by tTG. Indeed, tTG-cross-linked normal fibrin contained products very similar to FXIIIa-cross-linked γK406R, with the exception of a small amount of γ-dimer present in the tTG-cross-linked normal fibrin sample, which was absent in either FXIIIa- or tTG-cross-linked γK406R (Figure 2). In γQ398N/Q399N/K406R fibrin clots, tTG readily cross-linked the α-chains to each other, but found no available residues for cross-linking on the γ-chains. In tTG-cross-linked fibrinogen samples, similar patterns were found as those observed in fibrin clots, but cross-linking occurred as expected at a slower rate, and there was no γ-dimer present in normal fibrin (data not shown). Generally, tTG preferentially cross-linked the α-chains rather than the γ-chains in all samples (Figure 2B). All tTG-cross-linked samples showed 2 bands of a molecular weight between 102 000 and 122 000 kDa, which were caused by impurities in the commercial tTG preparation, as they were not evident in blots with fibrinogen-specific antibodies, but were present on SDS-PAGE of tTG alone (data not shown).
Cross-linking of fibrinogen and fibrin by tTG. Comparison of FXIIIa (A) and guinea pig tTG-cross-linked products (B). Lanes 1 and 4: normal recombinant fibrin; lanes 2 and 5: γQ398N/Q399N/K406R; lanes 3 and 6: γK406R. Bands are labeled as in Figure 1. With the exception of a faint γ-dimer band, the cross-linking pattern in tTG-cross-linked normal recombinant fibrin is similar to that of FXIIIa- and tTG-cross-linked γK406R. Nature of the cross-linking bands was identified by Western blotting.
Cross-linking of fibrinogen and fibrin by tTG. Comparison of FXIIIa (A) and guinea pig tTG-cross-linked products (B). Lanes 1 and 4: normal recombinant fibrin; lanes 2 and 5: γQ398N/Q399N/K406R; lanes 3 and 6: γK406R. Bands are labeled as in Figure 1. With the exception of a faint γ-dimer band, the cross-linking pattern in tTG-cross-linked normal recombinant fibrin is similar to that of FXIIIa- and tTG-cross-linked γK406R. Nature of the cross-linking bands was identified by Western blotting.
Stiffness of clots made from the fibrinogen variants
The storage modulus G′, which is as a measure of clot rigidity, was comparable for all samples before addition of FXIII (Table 1). After cross-linking by FXIIIa, clots made from the fibrinogen γ-chain variants markedly differed in stiffness. Cross-linking by FXIIIa increased stiffness of clots in all samples, but to a different extent: normal samples demonstrated the most drastic increase in stiffness, with G′ increasing 3.5-fold (Table 1). In clots made from the γQ398N/Q399N/K406R fibrinogen variant, where cross-linking of the γ-chain is completely abolished as shown above, G′ increased 2.5-fold, indicating that cross-linking of the α-chain alone is not solely responsible for clot stiffness, and that γ-dimer and γ-multimer formation must play a role. The smallest increase (1.5-fold) induced by FXIIIa cross-linking was achieved in γK406R clots (Table 1), indicating that α-γ cross-links are least effective in enhancing clot rigidity.
Fibrin clot stiffness with and without cross-linking
| . | Normal recombinant fibrinogen . | γK406R . | γQ398N/Q399N/K406R . | Plasma fibrinogen . |
|---|---|---|---|---|
| Number | 3 | 3 | 3 | 3 |
| G′, dyne/cm2 ± SD, before cross-linking | 28.3 ± 2.9 | 18.1 ± 2.1 | 16.7 ± 1.6 | 23.5 ± 2.2 |
| G′, dyne/cm2 ± SD, after cross-linking | 98.9 ± 17.6*† | 29.9 ± 2.3†‡ | 41.2 ± 2.1† | 64.4 ± 2.9† |
| Fold increase of G′ after cross-linking | 3.5× | 1.5× | 2.5× | 2.8× |
| . | Normal recombinant fibrinogen . | γK406R . | γQ398N/Q399N/K406R . | Plasma fibrinogen . |
|---|---|---|---|---|
| Number | 3 | 3 | 3 | 3 |
| G′, dyne/cm2 ± SD, before cross-linking | 28.3 ± 2.9 | 18.1 ± 2.1 | 16.7 ± 1.6 | 23.5 ± 2.2 |
| G′, dyne/cm2 ± SD, after cross-linking | 98.9 ± 17.6*† | 29.9 ± 2.3†‡ | 41.2 ± 2.1† | 64.4 ± 2.9† |
| Fold increase of G′ after cross-linking | 3.5× | 1.5× | 2.5× | 2.8× |
Stiffer than cross-linked γK406R, γQ398N/Q399N/K406R, and plasma-purified fibrin by one-way ANOVA (P < .05).
Stiffer after cross-linking by independent t test (P < .05).
Less stiff than plasma and normal recombinant fibrinogen (P < .05).
Macroscopic and microscopic evaluation of fibrin clot morphology
No striking differences could be detected in the overall morphologic appearance of the clots before and after cross-linking, using macroscopic (turbidity) techniques and microscopic (scanning electron microscopy). Macroscopic analyses did not reveal significant differences in fibrin polymerization rate, rate of protofibril formation, or clot morphology (data not shown). In contrast, microscopic measurements of non–cross-linked fibers revealed that, while there was no difference between normal and γK406R clots, γQ398N/Q399N/K406R fibers were characterized by a slightly larger fiber diameter (Table 2). Fiber diameter in normal fibrin decreased subtly with cross-linking, in accordance with previous reports in the literature.21 In both γK406R and γQ398N/Q399N/K406R samples, cross-linking led to an increase in fiber diameter (Table 2).
Fiber diameter with and without FXIIIa cross-linking (determined by scanning electron microscopy)
| . | Recombinant normal . | γK406R . | γQ398N/Q399N/K406R . | |||
|---|---|---|---|---|---|---|
| Diameter (± SD), nm . | n . | Diameter (± SD), nm . | n . | Diameter (± SD), nm . | n . | |
| Non–cross-linked | 146 (23) | 600 | 145 (26) | 600 | 167 (28)† | 800 |
| Cross-linked | 137 (31)* | 800 | 168 (30)‡ | 800 | 184 (22)‡ | 800 |
| . | Recombinant normal . | γK406R . | γQ398N/Q399N/K406R . | |||
|---|---|---|---|---|---|---|
| Diameter (± SD), nm . | n . | Diameter (± SD), nm . | n . | Diameter (± SD), nm . | n . | |
| Non–cross-linked | 146 (23) | 600 | 145 (26) | 600 | 167 (28)† | 800 |
| Cross-linked | 137 (31)* | 800 | 168 (30)‡ | 800 | 184 (22)‡ | 800 |
Decrease in fiber diameter after cross-linking (P < .05).
Larger fiber diameter compared with non–cross-linked recombinant normal and γK406R fibrinogen (P < .05).
Increase in fiber diameter after cross-linking (P < .05).
Fibrin clot lysis
We investigated rates of fibrinolysis by following loss of turbidity following overlay of fibrin clots with a mixture of tissue plasminogen activator and plasminogen. Using the methods and conditions used, we did not observe differences in lysis rates between normal and variant fibrins, or between cross-linked and non–cross-linked samples. Subsequent analysis of the lysed samples on SDS-PAGE indicated complete lysis of the very high molecular weight multimers in all variants, although the pattern and composition of lysis products differed significantly for the cross-linked normal and γK406R and γQ398N/Q399N/K406R variants (data not shown).
Discussion
Previous studies that examined the role of γ-chain cross-linking in fibrin clot function in the absence of α-chain cross-linking made use of molecules with truncated α-chains (with all α-chain cross-linking residues removed)12 or patient IgG that inhibited formation of high molecular weight cross-linking products while leaving γ-dimer formation intact (in combination with total FXIII inhibition).11 Here we have taken the opposite and complementary approach, by specifically removing through site-directed mutagenesis the γ-chain residues involved in the transglutaminase reaction. Using these variants, we were able for the first time to directly characterize the effects of γ-chain cross-linking on fibrin function, without potential interference of an additional element such as an antibody, or deletion of substantial parts of the molecule.
We have found that substitution of the only γ lysine residue (γK406R) involved in the cross-linking process eliminated the production of γ-dimers due to cross-linking of γK406 to γQ399 or γQ398. However, we also found that FXIIIa produced a number of α-γ hybrid cross-links in this variant by cross-linking the 2 acceptor sites γQ398 and/or Q399 to lysine residues in the α-chain. Murthy et al20 found that in fibrinogen that was cross-linked with red cell transglutaminase, γQ398 and Q399 were cross-linked to AαK413 and K418, allowing for 4 possible conformations in hybrid α-γ cross-links: AαK413-γQ398, AαK413-γQ399, AαK418-γQ398, and AαK418-γQ399. When we cross-linked γK406R with FXIIIa or tTG, similar high molecular weight products could be observed, whereas in normal fibrin the cross-linking pattern was clearly different between FXIIIa and tTG. These findings suggest that in γK406R fibrinogen, FXIIIa uses the same combination of γQ398/399 and AαK413/418 as tTG, leading to preferential cross-linking of the γ- to the α-chain of fibrin. FXIII and tTG likely have different in vivo roles, which may require different cross-linking patterns. FXIII is important during the initial stage of clot formation, which requires a fibrin network that is able to seal the circulation at a site of injury, and withstand high pressure from the circulating blood. The role of tTG-cross-linked fibrinogen is not yet fully understood. tTG-cross-linked fibrinogen has been found in the fibrous cap of atherosclerotic lesions, a process that may not require the same physical properties as the creation of a hemostatic plug.22
We have also shown that FXIIIa-dependent γ-chain cross-linking contributes significantly to clot stiffness, in particular through γ-dimer formation, which maximizes clot stiffness. Collet et al12 measured stiffness in clots formed from fibrinogen with a truncation at Aα chain residue 251, a variant without the C-terminal flexible αC region, where the α-chain FXIII-cross-linking sites are located, hence only allowing for γ-chain cross-linking. Fibrin clots formed with this variant showed a 1.5 × increase in clot stiffness after FXIII cross-linking compared with a 2.5 × increase in normal fibrin samples. Using our modified fibrinogen molecules, we found a pronounced difference in stiffness between our recombinant normal samples (3.5 × increase) and the γK406R variant (1.5 × increase), indicating a major contribution of γ-chain cross-linking in determining viscoelastic properties of clots. Some of the differences between the results of Collet et al and our current results may be caused by effects of the absence of the αC regions in their study, which may contribute to changes in lateral aggregation, fibrin structure, and hence stiffness of the clot. We were able to demonstrate that γ-γ cross-linking is a prerequisite for the production of high molecular weight formations that maximally enhance clot stiffness, confirming the hypothesis of Ryan et al11 that the ligation of γ-chains is likely to provide a structural framework for the later-developing higher-order ligated chain species to enhance network stiffness.11 Interestingly, when we eliminated all γ-chain cross-linking sites (γQ398N/Q399N/K406R), the increase in stiffness induced by cross-linking (2.5 ×) was indeed less when compared with recombinant normal fibrinogen (3.5 ×), but more than that observed in γK406R (1.5 ×). As the main difference between the γQ398N/Q399N/K406R and the γK406R variants is the formation of α-γ hybrid cross-links in the latter, these data suggest that α-γ cross-links are least effective in increasing the stiffness of the clot. We therefore conclude that the observed increase in stiffness following γ-dimer formation in normal fibrin clots must be due to formation of γ-multimers and αn-polymers, rather than αpγq-hybrid cross-links. Our data further understanding of the basic mechanisms involved in determining the mechanical properties of fibrin, although further studies are required to elucidate the processes determining the mechanical properties of plasma clots and thrombi.23 The importance of this is highlighted by evidence from epidemiologic studies demonstrating a relationship between cardiovascular events and clot mechanical properties.24,25 Furthermore, the striking effects of γ- and α-chain cross-linking on the decrease of the irreversible changes that occur after the release of stress,26 as well as the extent of increase in the fibrin period on stretching,27 have yet to be explored with these mutants.
In addition to viscoelastic properties, we also measured the influence of cross-linking patterns (γ-γ, α-γ, and α-α) on fibrin structure and fibrinolysis. We found the contribution of cross-linking patterns to clot morphology was modest, and we did not find any change in fibrinolysis rates between variants. The relationship between cross-linking and fibrinolysis has been the focus of many studies with conflicting interpretation regarding the contribution of α-chain or γ-chain multimerization or cross-linking per se on fibrinolysis rates.28-30 Using our recombinant variants and the turbidity plate assay, we observed that during early cross-linking (> 60 minutes), FXIII cross-linking did not increase fibrinolysis time. While we appreciate that this reaction time is relatively short to investigate the effects of complete cross-linking on fibrinolysis rates, our data in principle support findings by Siebenlist and Mosesson that there was no directly measurable relationship of resistance to fibrinolysis and the degree of α-chain cross-linking, and the formation of γ-dimers as they occur early on in clot formation did not extend fibrinolysis time.9
We conclude that γ-dimer formation is essential to generate a pattern of cross-linking by FXIIIa that maximally enhances rigidity in fibrin clots. Formation of α-γ hybrid cross-links, similar to those generated by tTG, is least efficient in strengthening the fibrin fibers. Different cross-linking patterns between the α- and γ-chains and the sequence in which they are formed play a major role in determining clot stiffness.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors wish to thank the British Heart Foundation for their generous support for these studies (PG/02/124/14 502), as well as the National Institutes of Health (HL30954 to J.W.W. and HL31048 to S.T.L.). The authors also would like to thank Mrs Li Fang Ping, Dr Susan Wilhelm, and Dr Bettina Bolliger-Stucki for their excellent advice in the production of recombinant fibrinogen.
Authorship
Contribution: K.F.S., A.M.C., P.J.G., and R.A.S.A. devised and managed the project; K.F.S. carried out the experimental procedures (apart from measurements of viscoelastic properties) and wrote the paper; J.W.W., I.C., and L.M. carried out measurements of viscoelastic properties; S.T.L. provided constructs and training on recombinant fibrinogen expression. All authors carried out editorial modifications and read and approved the final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter J. Grant, Academic Unit of Molecular Vascular Medicine, Leeds Institute for Genetics, Health and Therapeutics, Clarendon Way, University of Leeds, Leeds LS2 9JT UK; e-mail: p.j.grant@leeds.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal