Abstract
Palladin was originally found up-regulated with NB4 cell differentiation induced by all-trans retinoic acid. Disruption of palladin results in neural tube closure defects, liver herniation, and embryonic lethality. Here we further report that Palld−/− embryos exhibit a significant defect in erythropoiesis characterized by a dramatic reduction in definitive erythrocytes derived from fetal liver but not primitive erythrocytes from yolk sac. The reduction of erythrocytes is accompanied by increased apoptosis of erythroblasts and partial blockage of erythroid differentiation. However, colony-forming assay shows no differences between wild-type (wt) and mutant fetal liver or yolk sac in the number and size of colonies tested. In addition, Palld−/− fetal liver cells can reconstitute hematopoiesis in lethally irradiated mice. These data strongly suggest that deficient erythropoiesis in Palld−/− fetal liver is mainly due to a compromised erythropoietic microenvironment. As expected, erythroblastic island in Palld−/− fetal liver was found disorganized. Palld−/− fetal liver cells fail to form erythroblastic island in vitro. Interestingly, wt macrophages can form such units with either wt or mutant erythroblasts, while mutant macrophages lose their ability to bind wt or mutant erythroblasts. These data demonstrate that palladin is crucial for definitive erythropoiesis and erythroblastic island formation and, especially, required for normal function of macrophages in fetal liver.
Introduction
Palladin is an actin cytoskeleton–associated protein that is colocalized with α-actinin in the dense regions and focal adhesions of stress fibers.1 It is known that palladin, myopalladin, and myotilin belong to a new family of actin cytoskeleton structural proteins.2 These proteins localize along filament actin at the dense regions and play a role in the dynamic regulation of filament actin. However, myopalladin and myotilin are expressed specifically in muscle tissue, while palladin is ubiquitously expressed.3,4 In vivo and in vitro studies have shown that palladin is actively involved in the regulation of actin cytoskeleton dynamics and cell–extracellular matrix (ECM) interaction.1,5,6
Originally, it was also proposed that palladin may play an important role in all-trans retinoic acid (ATRA)–induced NB4 cell differentiation based on the fact that palladin can be highly induced in NB4 cells upon ATRA treatment.7 Unexpectedly, gene-targeting experimentation has shown that disruption of palladin in mice results in cranial neural tube closure defect, fetal liver herniation, and embryonic lethality before E15.5, implicating a crucial role of palladin in mouse embryogenesis.5 Palld−/− embryos mainly exhibit 2 apparent phenotypes: cranial neural tube closure defect and liver herniation.5 The reason palladin disruption leads to embryonic lethality remains unclear. Up to now, it has been reported that mutations of about 80 genes can lead to neural tube closure defects as shown by gene-targeting experiments.8 Some mutant mice, such as transcription factor AP-1 knockout mice, can live until birth although cranial neural tube closure defect occurs.9 Fetal liver herniation always results from the closure defect of the dorsal ventral body wall. Some mutations also cause fetal liver herniation, but not embryonic lethality, such as ROCK-I (Rho-associated kinase I) mutation in mice.10
Here we further show that palladin-deficient mouse embryos exhibit severe anemia between E13.5 and E14.5. This anemic state may contribute to the phenotype of embryonic lethality. It is well known that embryonic hematopoiesis first initiates at E7.5 from the blood island of the yolk sac.11 This is primitive hematopoiesis, which produces erythrocytes with a nucleus. The stem cells of primitive hematopoiesis have limited capability in hematopoietic reconstitution. At E8.5, definitive hematopoietic stem cells (HSCs) arise from the paraaortic splanchnopleura/aorta-gonads-mesonephros (P-Sp/AGM) region.12-15 These HSCs have the full potential to reconstitute hematopoiesis in lethally irradiated mice. At E10.5, HSCs migrate from P-Sp/AGM to fetal liver, where definitive hematopoiesis initiates.
Previous study has shown that some actin cytoskeleton regulatory proteins are critically involved in hematopoiesis, such as Wiskott-Aldrich syndrome protein (WASP).16 But the role of palladin in hematopoiesis, especially in fetal liver erythropoiesis, remains unclear. Our study indicates that palladin is crucial for definitive erythropoiesis and erythroblastic island formation and especially is required for normal function of macrophages in fetal liver. To our knowledge, palladin is the first actin cytoskeleton regulatory protein that is involved in fetal liver erythropoiesis.
Materials and methods
Mice
Mice containing a heterozygous deletion of palladin were maintained on 129SvJ background. The procedures described were approved by the Animal Use and Care Committee of Shanghai Jiao Tong University School of Medicine.
Hematologic analysis of embryonic blood
Hematocrit was measured using micropipette (Microcaps; Drummond Scientific, Broomall, PA). Blood for red cell counts was collected by bleeding decapitated embryos into phosphate-buffered saline (PBS), and counts were done using a hemocytometer. Fetal peripheral blood smears were prepared by cytospin technique and then stained with Wright-Giemsa.
Immunostaining and flow cytometric analysis
Freshly isolated fetal liver cells simultaneously stained for FITC-CD71, PE-Ter119, APC–annexin V, and 7-AAD according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Flow cytometry was carried out with FACSCalibur machine (BD, San Jose, CA), and 7-AAD–positive cells were gated out in subsequent differentiation and apoptosis analysis.
TUNEL
Fluorescent terminal dUTP nick-end labeling (TUNEL) assays were performed using an in situ cell death detection kit (fluorescein based) according to the manufacturer's protocol (Promega, Madison, WI). Images were taken with an Olympus BX51 fluorescent microscope (Olympus, Tokyo, Japan).
Reconstitution of lethally irradiated mice
The fetal liver of E13.5 embryos was dissociated in sterile PBS. Trypan blue–excluding nucleated cells (5 × 105) were injected intravenously into 129SvJ recipient mice (8 to 10 weeks old). These mice were lethally irradiated in 2 doses of 4.8 Gy separated by 3 hours before receiving the donor cells. After 2 months, the peripheral blood of recipient mice was analyzed with a hematology analyzer (Beckman Coulter, Fullerton, CA). Cells from peripheral blood, bone marrow, and spleen were stained with fluorescence-conjugated antibodies to determine the proportion of lympholoid, myeloid, and erythroid lineages. The genomic DNA was extracted from 106 nucleated peripheral blood cells or 2 × 106 spleen or bone marrow cells. Donor-derived hematopoietic cells were determined by 30 cycles of polymerase chain reaction (PCR) specific for mutant or wild-type (wt) allele.
Transmission electron microscopy analysis
Wt or Palld−/− fetal livers were fixed in 2% glutaraldehyde overnight. Then the fetal livers were postfixed in 1% OsO4 for 2 hours, dehydrated in a graded series of ethanol, transferred to propylene oxide, and embedded in Epon (Emicron, New Delhi, India) according to standard procedures. Ultrathin sections were cut and then examined using a Philips CM 120 transmission electron microscope (Eindhoven, The Netherlands).
Isolation of native erythroblastic islands and reconstitution of heterologous erythroblastic islands
Clusters composed of fetal liver macrophages and erythroblasts were isolated according to previously published protocol.17 Briefly, fetal liver single cells obtained by enzyme digestion were allowed to attach to glass coverslips for 20 minutes at 37°C in 5% CO2. Cells were then flooded with medium and incubated further for 4 hours. Adherent macrophages with attached erythroid clusters (“native” clusters) were obtained by dipping coverslips in RPMI to remove nonadherent cells. To strip erythroblasts from macrophages, cells were incubated in PBS lacking calcium and magnesium for 10 minutes. Adherent macrophages were allowed to respread in complete medium for 2 hours before receiving fresh erythroblasts (reconstituted clusters). Finally, the reconstituted clusters were dipped to remove the unbound cells before being processed for double F4/80-Ter119 immunofluorescences using standard procedures.
Statistical analysis
Data were represented as mean plus and minus a standard deviation (SD) or plus and minus a standard error of the mean (SEM). Statistical significance between any 2 groups was determined by the 2-tailed Student t test. P values less than .05 were considered significant.
Results
Palld−/− embryos show severe anemia due to impairment of definitive erythropoiesis
We have previously constructed palladin knockout mice through homologous recombination. It was found that palladin disruption results in cranial neural tube closure defects, fetal liver herniation, and embryonic lethality.5 To further understand the reason palladin disruption causes embryonic lethality, we collected and genotyped embryos from E11.5 to E15.5 to delineate the time when homozygous mutants die. It was found that Palld−/− embryos began to die at E12.5 and all died at E15.5 (Figure 1A). The hematopoietic system is critical for embryonic development. It was noted that all alive Palld−/− embryos at E13.5 and E14.5 appear pale compared with wt embryos (Figure 1B). Therefore, we checked the hematocrit value of wt and Palld−/− embryos and found that hematocrit value of alive mutants was about half of that in wt embryos at E13.5 (Figure 1C). Fetal erythropoiesis shifts from yolk sac to fetal liver at E10.5. In yolk sac the product of erythropoiesis is primitive erythrocytes, namely, nucleated erythrocytes (eryPs). However, the major product in the fetal liver is enucleated definitive erythrocytes (eryDs). Palladin-deficient mice died from E12.5 to E15.5, a period when definitive erythropoiesis really works, and displayed severe anemia. We further stained fetal peripheral blood smear with Wright-Giemsa and counted the ratio of nucleated and enucleated red blood cells in wt and Palld−/− mice. We found a dramatic reduction of enucleated red blood cells in mutant mice while the nucleated primitive erythrocytes remain constant in wt and Palld−/− mice. At E13.5, enucleated erythrocytes in wt account for about 40% of peripheral RBCs but only 5% in mutant mice. At E14.5, 75% of enucleated RBCs in wt compared with 10% in Palld−/− mice (Figure 1D,E). These findings suggest that disruption of palladin results in severe fetal anemia, mainly due to impairment of definitive erythropoiesis. In addition, marked edema can be easily discerned in the dorsal part of all alive E14.5 Palld−/− embryos as a result of severe anemia. Embryonic lethality due to disruption of palladin is mainly caused by embryonic anemia. However, we still cannot rule out other possibilities, such as neural tube closure defects and other unknown abnormalities, which may also cause embryonic lethality.
Palladin-deficient embryos show severe anemia. (A) Viability of Palld−/− embryos. The percentage of viable Palld−/− embryos (of the total viable embryos collected) is shown as a function of increasing gestational age. All embryos were derived from Palld+/− intercrosses, so 25% would be expected to be Palld−/−. The analysis was performed on a pure 129SvJ background. At each time point, 40 to 200 embryos were analyzed. (B) An anemic Palld−/− E13.5 embryo and its wt control. (C) Hematocrit values for wt (n = 5) and Palld−/− (n = 5) E13.5 embryos. Error bar represents plus and minus a standard deviation (SD); **P < .01. (D) Peripheral blood counts of wt and Palld−/− E13.5 and E14.5 mouse embryos. Palld−/− embryos exhibit decreased definitive erythrocytes (eryDs) but not primitive erythrocytes (eryPs). (E) Wright-Giemsa staining of peripheral blood smears of wt and Palld−/− embryos at E13.5; Palld−/− peripheral blood contains much less enucleated red blood cells. Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 40×/0.75 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments, Sterling Heights, MI), and imported into SPOT software (Diagnostic Instruments). Original magnification, × 400.
Palladin-deficient embryos show severe anemia. (A) Viability of Palld−/− embryos. The percentage of viable Palld−/− embryos (of the total viable embryos collected) is shown as a function of increasing gestational age. All embryos were derived from Palld+/− intercrosses, so 25% would be expected to be Palld−/−. The analysis was performed on a pure 129SvJ background. At each time point, 40 to 200 embryos were analyzed. (B) An anemic Palld−/− E13.5 embryo and its wt control. (C) Hematocrit values for wt (n = 5) and Palld−/− (n = 5) E13.5 embryos. Error bar represents plus and minus a standard deviation (SD); **P < .01. (D) Peripheral blood counts of wt and Palld−/− E13.5 and E14.5 mouse embryos. Palld−/− embryos exhibit decreased definitive erythrocytes (eryDs) but not primitive erythrocytes (eryPs). (E) Wright-Giemsa staining of peripheral blood smears of wt and Palld−/− embryos at E13.5; Palld−/− peripheral blood contains much less enucleated red blood cells. Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 40×/0.75 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments, Sterling Heights, MI), and imported into SPOT software (Diagnostic Instruments). Original magnification, × 400.
Increased apoptosis and partial differentiation blockage of erythroblasts after palladin disruption
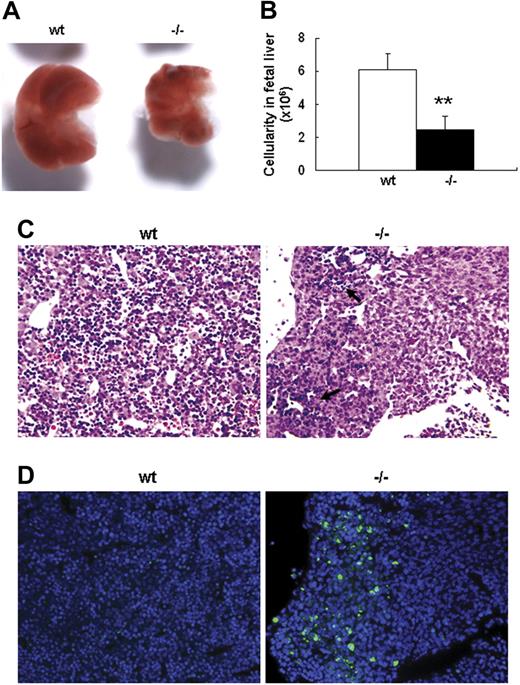
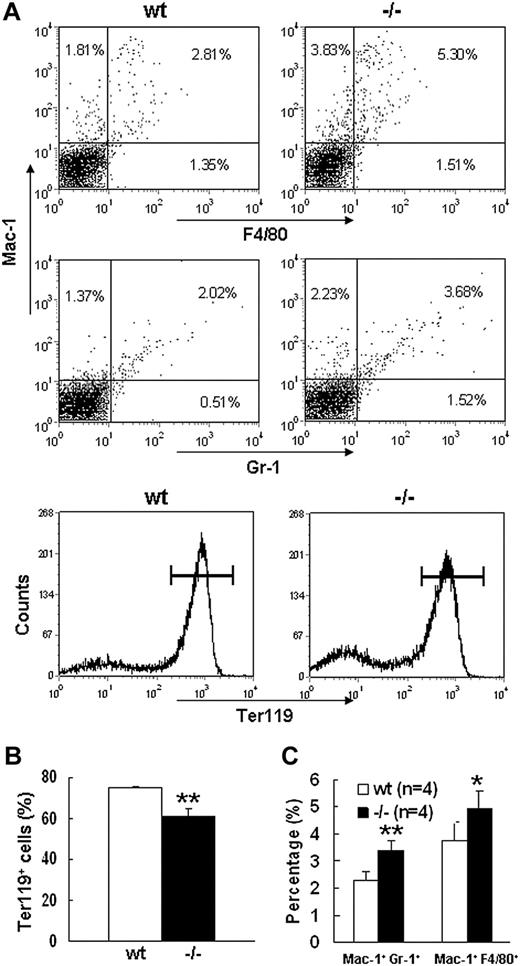
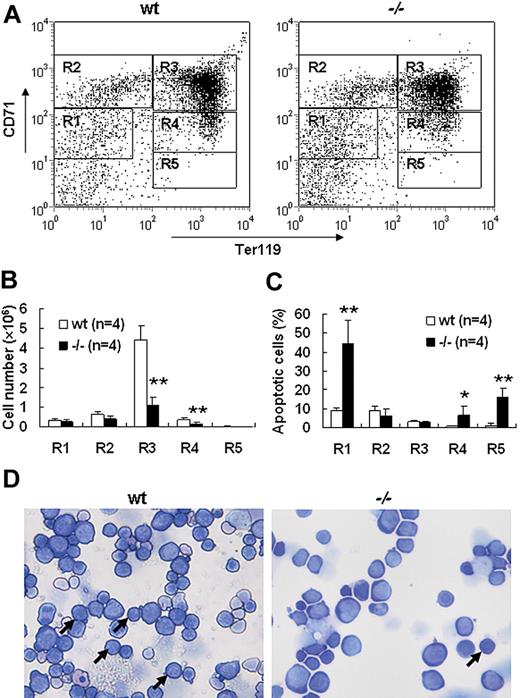
Severe defect in definitive erythropoiesis found in Palld−/− embryos was accompanied by significant fetal liver atrophy (Figure 2A). The total cellularity of mutant fetal liver was about half of wt counterpart at E13.5 (Figure 2B). We further compared hematoxylin and eosin (H&E)–stained sections of wt and Palld−/− fetal livers. A lot of pyknotic nuclei, which are indicative of apoptosis, were found in Palld−/− fetal liver (Figure 2C). TUNEL assay showed that palladin disruption results in significant increased apoptosis (Figure 2D). More interestingly, the apoptosis mainly occurs in the region where hematopoiesis takes place, while in the region devoid of hematopoiesis apoptosis does not occur (Figure 2C,D). This suggests that hematopoietic cells in Palld−/− fetal liver are undergoing apoptosis. To characterize the hematopoiesis and apoptosis that occurred in Palld−/− fetal liver, flow cytometry assay was performed. We found a decreased ratio of erythroid cells accompanied by an increased ratio of macrophages and myeloid cells in Palld−/− fetal liver (Figure 3A-C). By using annexin V, CD71, and Ter119, we further analyzed the apoptosis and differentiation state of erythroid cells. Erythroid cells can be classified into 5 populations using cell surface marker CD71 and Ter119—termed R1, R2, R3, R4 and R5—with more mature differentiation state.18 We observed a partial differentiation blockage of erythroblasts in Palld−/− fetal liver, evidenced by an increase in the number of less mature erythroblasts (R1, R2) and a marked reduction of more mature erythroblasts (R3 to R5) (Figure 4A,B). A similar result was also observed on fetal liver cell cytospin slides stained with Wright-Giemsa (Figure 4D). Apoptosis analysis using 4-color flow cytometry further showed that more annexin V–positive cells were detected in R1, R4, and R5 populations compared with wt counterparts (P > .05), whereas among R2 and R3 populations the annexin V–positive cells were comparable to wt cells (Figure 4C).
Defective fetal liver definitive erythropoiesis in palladin-deficient embryos. (A) Typical images of wt and Palld−/− E13.5 fetal livers. (B) The cellularity of wt and Palld−/− E13.5 fetal livers. Error bar represents plus and minus a SD; **P < .01. (C) H&E staining of wt and Palld−/− fetal liver sections. (D) TUNEL assay shows increased apoptosis in Palld−/− fetal liver at the same location as in panel C. Note that the apoptosis mainly occurs in the region where hematopoiesis takes place (arrows). (C,D) Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 10×/0.30 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). In panel D, DAPI (nuclei) and FITC (apoptotic cells) fluorescence are shown. Original magnification, × 100.
Defective fetal liver definitive erythropoiesis in palladin-deficient embryos. (A) Typical images of wt and Palld−/− E13.5 fetal livers. (B) The cellularity of wt and Palld−/− E13.5 fetal livers. Error bar represents plus and minus a SD; **P < .01. (C) H&E staining of wt and Palld−/− fetal liver sections. (D) TUNEL assay shows increased apoptosis in Palld−/− fetal liver at the same location as in panel C. Note that the apoptosis mainly occurs in the region where hematopoiesis takes place (arrows). (C,D) Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 10×/0.30 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). In panel D, DAPI (nuclei) and FITC (apoptotic cells) fluorescence are shown. Original magnification, × 100.
Flow cytometric analysis of E13.5 wt andPalld−/−fetal liver cells. (A) Flow cytometric profiles of wt and Palld−/− E13.5 fetal liver cells labeled with Mac-1/Gr-1 (granulocyte), Mac-1/F4/80 (myelomonocyte), or Ter119 (erythrocyte). Values shown are percentages of each cell population among total cells. Horizontal lines in the bottom panels represent Ter119+ cells. (B) Quantification of Ter119+ cells in wt and Palld−/− fetal livers. (C) Quantification of Mac-1+ Gr-1+ and Mac-1+F4/80+ cells in wt and Palld−/− fetal livers. Error bar represents plus and minus a SD; **P < .01; *P < .05.
Flow cytometric analysis of E13.5 wt andPalld−/−fetal liver cells. (A) Flow cytometric profiles of wt and Palld−/− E13.5 fetal liver cells labeled with Mac-1/Gr-1 (granulocyte), Mac-1/F4/80 (myelomonocyte), or Ter119 (erythrocyte). Values shown are percentages of each cell population among total cells. Horizontal lines in the bottom panels represent Ter119+ cells. (B) Quantification of Ter119+ cells in wt and Palld−/− fetal livers. (C) Quantification of Mac-1+ Gr-1+ and Mac-1+F4/80+ cells in wt and Palld−/− fetal livers. Error bar represents plus and minus a SD; **P < .01; *P < .05.
Increased apoptosis and partial differentiation blockage of erythroblasts after palladin disruption. (A) Typical flow cytometric profiles of wt and Palld−/− fetal liver single cells stained with CD71 and Ter119 are shown. Gates from R1 to R5 are set as indicated. (B) Comparison of wt and Palld−/− fetal livers in the ratio of R1 to R5. (C) The ratio of annexin V–positive cells from R1 to R5 was compared in wt and Palld−/− fetal livers. (D) Wt and Palld−/− fetal liver single-cell cytospins were stained with Wright-Giemsa; arrows indicate the R3 population. Images were viewed with an Olympus BX51 microscope with an Olympus UplanFl 20×/0.50 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). Note that Palld−/− fetal liver has a decreased R3 population. Error bar represents plus and minus a SD; **P < .01; *P < .05. Original magnification, × 200.
Increased apoptosis and partial differentiation blockage of erythroblasts after palladin disruption. (A) Typical flow cytometric profiles of wt and Palld−/− fetal liver single cells stained with CD71 and Ter119 are shown. Gates from R1 to R5 are set as indicated. (B) Comparison of wt and Palld−/− fetal livers in the ratio of R1 to R5. (C) The ratio of annexin V–positive cells from R1 to R5 was compared in wt and Palld−/− fetal livers. (D) Wt and Palld−/− fetal liver single-cell cytospins were stained with Wright-Giemsa; arrows indicate the R3 population. Images were viewed with an Olympus BX51 microscope with an Olympus UplanFl 20×/0.50 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). Note that Palld−/− fetal liver has a decreased R3 population. Error bar represents plus and minus a SD; **P < .01; *P < .05. Original magnification, × 200.
To further address whether palladin is involved in HSC differentiation and proliferation, we also tested the ability in colony formation in vitro of hematopoietic progenitors in Palld−/− fetal liver and yolk sac. It was shown that palladin-deficient hematopoietic progenitor cells can form erythroid and myeloid colonies. There were no significant differences between wt and mutant hematopoietic progenitor cells from either fetal liver or yolk sac in terms of number and size of colonies tested (Figure S1A,B; available on the Blood website; see the Supplemental Materials link at the top of the online article). These data suggest that impaired erythropoiesis found in Palld−/− embryos might be due to defects in the erythropoietic microenvironment rather than intrinsic defects of hematopoietic progenitor cells.
HSCs in Palld−/− fetal liver have the ability to reconstitute hematopoiesis in lethally irradiated mice
To distinguish the defects in either the erythropoietic microenvironment or hematopoietic progenitor cells in Palld−/− fetal liver, we conducted fetal liver HSC reconstitution assay. After lethal irradiation, the recipient mice were underwent transplantation with 5 × 105 fetal liver cells isolated from wt and Palld−/− E13.5 embryos via tail venous injection. After 2 months, all control mice that did not receive fetal liver cells died. However, half of recipients reconstituted with both wt and mutant fetal liver cells survived (Figure 5A). The genotyping analysis showed that the targeted band from the mutant allele was dominantly amplified from genomic DNA extracted from peripheral blood, bone marrow, and spleen of mutant cell recipients (Figure 5B). The peripheral blood, bone marrow cells, and splenocytes of recipients were analyzed. No differences between wt and Palld−/− HSC recipients in proportion of Mac-1+ Gr-1+ cells and Ter119+ cells were found (Figure 5C,D). These results indicate that Palld−/− HSCs have the ability to reconstitute the hematopoiesis in lethally irradiated mice.
Palld−/−fetal liver hematopoietic stem cells (HSCs) have the ability to reconstitute the hematopoiesis of lethally irradiated mice. (A) Equal numbers of wt or Palld−/− fetal liver cells were injected into lethally rradiated recipient mice, and the survival ratio of recipient mice was measured for 6 weeks. Nine mice received wt fetal liver cells, and 6 mice received Palld−/− fetal liver cells. (B) Genotyping of peripheral blood (PB), bone marrow (BM), and spleen (SP) of recipient mice 2 months after reconstitution shows the dominance of fetal liver–derived cells in the hematopoietic organs of Palld−/− HSC-reconstituted mice. W1 to W3 indicate the recipient mice receiving transplants with wt fetal liver cells; R1 to R5, the recipients of Palld−/− fetal liver cells. (C,D) Proportion of Mac-1+ Gr-1+ (C) and Ter119+ (D) cells among all nucleated peripheral blood cells (PB), bone marrow cells (BM), or spleen cells (SP) in wt and Palld−/− fetal liver cells reconstituted mice. No significant difference was observed between wt and Palld−/− fetal liver cells reconstituted mice. Error bar represents plus and minus a SD.
Palld−/−fetal liver hematopoietic stem cells (HSCs) have the ability to reconstitute the hematopoiesis of lethally irradiated mice. (A) Equal numbers of wt or Palld−/− fetal liver cells were injected into lethally rradiated recipient mice, and the survival ratio of recipient mice was measured for 6 weeks. Nine mice received wt fetal liver cells, and 6 mice received Palld−/− fetal liver cells. (B) Genotyping of peripheral blood (PB), bone marrow (BM), and spleen (SP) of recipient mice 2 months after reconstitution shows the dominance of fetal liver–derived cells in the hematopoietic organs of Palld−/− HSC-reconstituted mice. W1 to W3 indicate the recipient mice receiving transplants with wt fetal liver cells; R1 to R5, the recipients of Palld−/− fetal liver cells. (C,D) Proportion of Mac-1+ Gr-1+ (C) and Ter119+ (D) cells among all nucleated peripheral blood cells (PB), bone marrow cells (BM), or spleen cells (SP) in wt and Palld−/− fetal liver cells reconstituted mice. No significant difference was observed between wt and Palld−/− fetal liver cells reconstituted mice. Error bar represents plus and minus a SD.
Palladin is crucial for erythroblastic island formation
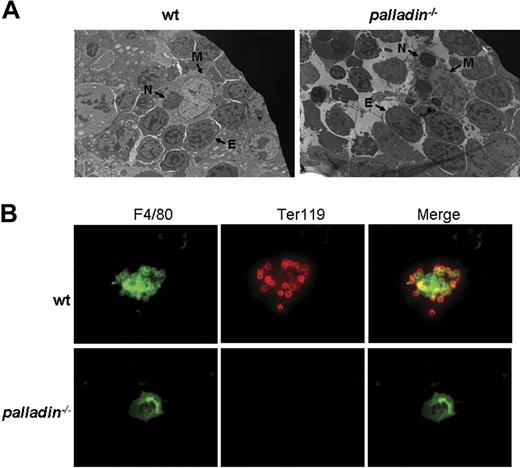
Normal proliferation and differentiation of Palld−/− progenitor cells or HSCs in vitro, as well as the fact that the mutant HSCs can reconstitute erythropoiesis in lethally irradiated mice, strongly suggest that the defects in definitive fetal liver erythropoiesis after palladin disruption may be caused by a compromised fetal liver erythropoietic microenvironment, which mainly consists of clusters of erythroblastic islands. The erythroblastic island is made up of central macrophage and surrounding erythroblasts.19 We used electron microscopy to analyze the morphology of erythroblastic island in Palld−/− fetal liver. In the wt erythroblastic island, a coherent connection between macrophage and erythroblasts was noticed. Compared with the wt erythroblastic island, the Palld−/− erythroblastic island is dispersed. Erythroblasts are separated from central macrophage, and some erythroblasts have pyknotic nuclei, which are indicative of apoptosis. Furthermore, it was also observed that several phagocytosed nuclei exist in mutant macrophage, suggesting that erythroblast enucleation and phagocytosis in macrophages deficient for palladin remains normal (Figure 6A).
Impaired fetal liver erythroblastic island formation after palladin disruption. (A) Transmission electron microscopy image of E13.5 wt and Palld−/− fetal livers. The erythroblastic island is composed of a central macrophage (M) and surrounding erythroblasts (E). In wt fetal liver, the connection between macrophage and erythroblasts is coherent, while this coherent connection is dispersed after palladin disruption. Note several phagocytosed nuclei (indicated as “N”) existing in mutant macrophage. (B) Native erythroblastic islands isolated from wt and Palld−/− fetal livers were double-immunostained with F4/80 (green) and TER 119 (red) as described in “Materials and methods.” F4/80 is macrophage-specific marker, and Ter119 is the marker for erythroblasts. Note that Palld−/− fetal liver cells could not form an erythroblastic island in this in vitro macrophage and erythroblast coculture assay. Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 40×/0.75 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). FITC (F4/80+ cells) and PE (Ter119+ cells) fluorescence are shown. Original magnifications, × 3000 (panel A) and × 400 (panel B).
Impaired fetal liver erythroblastic island formation after palladin disruption. (A) Transmission electron microscopy image of E13.5 wt and Palld−/− fetal livers. The erythroblastic island is composed of a central macrophage (M) and surrounding erythroblasts (E). In wt fetal liver, the connection between macrophage and erythroblasts is coherent, while this coherent connection is dispersed after palladin disruption. Note several phagocytosed nuclei (indicated as “N”) existing in mutant macrophage. (B) Native erythroblastic islands isolated from wt and Palld−/− fetal livers were double-immunostained with F4/80 (green) and TER 119 (red) as described in “Materials and methods.” F4/80 is macrophage-specific marker, and Ter119 is the marker for erythroblasts. Note that Palld−/− fetal liver cells could not form an erythroblastic island in this in vitro macrophage and erythroblast coculture assay. Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 40×/0.75 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). FITC (F4/80+ cells) and PE (Ter119+ cells) fluorescence are shown. Original magnifications, × 3000 (panel A) and × 400 (panel B).
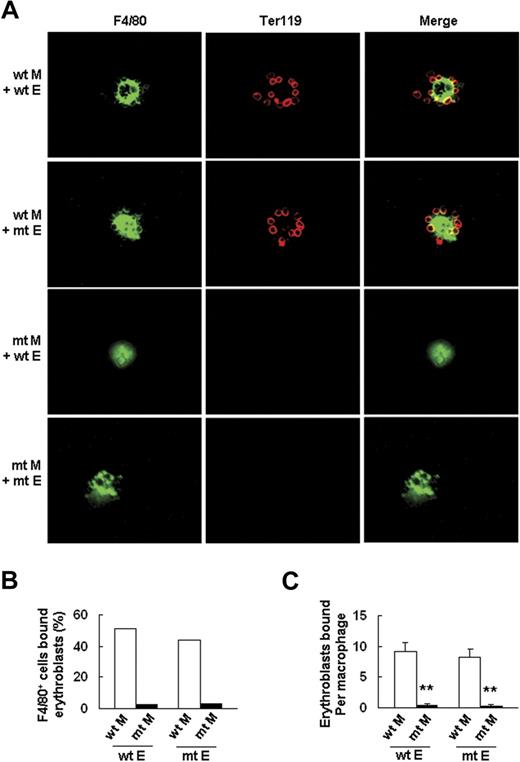
To further confirm this finding, we tested the ability of wt and Palld−/− fetal liver cells to form erythroblastic islands according to a previously described method.21 The results showed that Palld−/− fetal liver cells have a significantly decreased ability to form an erythroblastic island in this macrophage and erythroblast coculture assay in vitro (Figure 6B). Reconstitution experiments were conducted to determine whether impaired erythroblastic island formation in the absence of palladin is due to an abnormality in macrophages, erythroblasts, or both. The results indicate that Palld−/− erythroblasts can form an erythroblastic island with wt macrophage, while Palld−/− macrophages plus wt erythroblasts are unable to form an erythroblastic island (Figure 7A-C). These results further demonstrate that the defects in erythropoiesis of Palld−/− embryos are mainly due to an abnormal fetal liver erythropoietic microenvironment, especially due to abnormal function of macrophages in forming erythroblastic islands.
Palld−/−macrophage, but notPalld−/−erythroblast, has an intrinsic defect in the formation of an erythroblastic island. (A) Reconstituted, homologous (wt macrophages/wt erythroblasts or mutant (mt) macrophages/mt erythroblasts), and heterologous (wt macrophages/mt erythroblasts or mt macrophages/wt erythroblasts) erythroblastic islands were immunostained with F4/80 (green) and TER119 (red) antibodies. The result shows that Palld−/− fetal liver macrophage has an intrinsic defect in the formation of an erythroblastic island. Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 40×/0.75 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). FITC (F4/80+ cells) and PE (Ter119+ cells) fluorescence are shown. (B) Percentage of F4/80+ cells that bound erythroblasts in erythroblastic island reconstitution assay. (C) Erythroblasts bound per macrophage in erythroblastic island reconstitution assay. For each combination, 20 macrophages were analyzed. M indicates macrophage; E, erythroblast. Error bar represents plus and minus a SEM; **P < .01. Original magnification, × 400 (A).
Palld−/−macrophage, but notPalld−/−erythroblast, has an intrinsic defect in the formation of an erythroblastic island. (A) Reconstituted, homologous (wt macrophages/wt erythroblasts or mutant (mt) macrophages/mt erythroblasts), and heterologous (wt macrophages/mt erythroblasts or mt macrophages/wt erythroblasts) erythroblastic islands were immunostained with F4/80 (green) and TER119 (red) antibodies. The result shows that Palld−/− fetal liver macrophage has an intrinsic defect in the formation of an erythroblastic island. Images were viewed with an Olympus BX51 microscope with an Olympus UPlanFl 40×/0.75 objective, captured with a SPOT RTKE cooled color CCD camera (Diagnostic Instruments), and imported into SPOT software (Diagnostic Instruments). FITC (F4/80+ cells) and PE (Ter119+ cells) fluorescence are shown. (B) Percentage of F4/80+ cells that bound erythroblasts in erythroblastic island reconstitution assay. (C) Erythroblasts bound per macrophage in erythroblastic island reconstitution assay. For each combination, 20 macrophages were analyzed. M indicates macrophage; E, erythroblast. Error bar represents plus and minus a SEM; **P < .01. Original magnification, × 400 (A).
Discussion
Our current study shows palladin, an actin cytoskeleton–associated protein, is an important regulator of fetal liver definitive erythropoiesis. Its disruption results in significant fetal anemia caused by impaired fetal liver definitive erythropoiesis. However, the mutant HSCs in fetal liver can reconstitute lethally irradiated mice and differentiate into different lineages in the methylcellulose culture system. These results suggest that palladin may regulate definitive erythropoiesis not through a cell-autonomous manner. Impairment of the fetal liver erythropoietic microenvironment may be the major cause of definitive erythropoiesis deficiency. Interestingly, macrophages isolated from wt mice can form erythroblastic islands with either wt or Palld−/− erythroblasts. While macrophages isolated from Palld−/− embryos are unable to form erythroblastic islands with wt or Palld−/− erythroblasts, demonstrating that intrinsic defects in Palld−/− macrophages but not erythroblasts are responsible for impaired erythroblastic island formation, consequently, definitive erythropoiesis deficiency. To further address whether recipient macrophages or donor defective macrophages contribute to the erythropoietic reconstitution in lethally irradiated mice, we performed an erythroblastic island formation assay with macrophages isolated from lethally irradiated wt mice. As expected, “recipient” macrophages, as ones from unirradiated mice, can form erythroblastic islands with Palld−/− erythroblasts (Figure S2.). Thus, we may conclude that Palld−/− fetal liver cells are able to reconstitute lethally irradiated mice mainly dependent on the recipient macrophages, major players in the erythropoietic microenvironment.
Palladin disruption leads to apoptosis of erythropoietic cells. The apoptotic cells can be observed only in the place where hematopoiesis occurs as shown by H&E and TUNEL assays. Using the electron microscope, we can also find that nucleated erythroblasts have fragmented nuclei and are undergoing apoptosis (data not shown). Apoptosis analysis using 4-color flow cytometry further shows that primitive erythroid progenitor cells (R1), chromatophilic and orthochromatophilic erythroblasts (R4), and late orthochromatophilic erythroblasts and reticulocytes (R5) are major resources of apoptotic cells. It is known that R4 and R5, as well as R3 populations, are characterized by the expression of a Ter119 marker. The erythroblastic island in fetal liver is composed of central macrophage and surrounding Ter119+ erythroblasts. The formation of such functional units is crucial for differentiation and survival of erythroblasts.19 Loss of the ability of macrophages to bind Ter119+ erythroblasts in the absence of palladin may be at least in part responsible for erythroblast apoptosis. In addition, the defects in the fetal liver erythropoietic microenvironment and increased apoptosis may also cause an inflammatory response, which may further contribute to the acceleration of apoptosis in the fetal liver, as shown by the regional distribution of apoptotic cells. Using semiquantitative reverse transcriptase (RT)–PCR, we found the up-regulation of inflammatory cytokine Tnf-α in Palld−/− fetal liver; as a consequence, the expression of RelB and RelC was also up-regulated in Palld−/− fetal liver (Figure S3).
An in vivo blockage of erythroid differentiation was observed after palladin disruption. The immature components R1 and R2 increased, and the more mature components, R3, R4, and R5, decreased. Previous study has shown that disruption of K-ras causes similar but more severe defects in erythroid maturation.20 Stem cell factor (SCF) disruption at a specific developmental stage can also cause this defect,21 but there was no difference in the expression of Scf and K-ras at the mRNA level in fetal liver after palladin disruption (Figure S3).
Our data indicate that the compromised fetal liver erythropoietic microenvironment is the major cause of impaired definitive erythropoiesis after palladin disruption. The microenvironment for fetal liver erythropoiesis includes (1) cytokines and chemokines secreted by nonhematopoietic stroma cells; (2) cell-cell interactions; and (3) cell-ECM interactions. We tested the expression of cytokines and chemokines in wt and palladin mutant fetal liver and could not find any differences in the expression of these genes at the mRNA level (Figure S3). As an actin cytoskeleton regulatory protein, palladin was supposed to play critical roles in the regulation of cell-cell and cell-ECM interactions.6 To this end, palladin may regulate the fetal liver hematopoietic microenvironment through the regulation of cell-cell or cell-ECM interactions fundamental for HSC development. Previous study has also demonstrated the importance of cell-cell and cell-ECM interactions for erythropoiesis. An in vitro study has shown that ECM protein fibronectin is necessary for terminal differentiation of murine erythroleukemia cells.22 The fibronectin receptor VLA-4 is also required for erythropoiesis.23 Using macrophages and erythroblasts in vitro culture assays we show that Palld−/− macrophages have intrinsic defects to bind erythroblasts to form an erythroblastic island, further demonstrating an important role of palladin in cell-cell interaction regulation.
Recently, it was reported that targeted disruption of mouse retinoblastoma (Rb) or erythroblast macrophage protein (Emp) causes the defects in formation of an erythroblastic island.19,24 Targeted disruption of Emp reveals the importance of actin cytoskeleton in the regulation of erythroblastic island formation.25 For this reason, we also checked the expression level of Emp at the mRNA level. No difference between wt and Palld−/− macrophages was found (data not shown). To further explore the potential mechanism underlying the defects of macrophages in erythroblastic island formation, we isolated and cultured macrophages from wt and Palld−/− fetal liver. Unfortunately, we were unable to show any differences in cell adhesion, morphology, expression, and distribution pattern of filament actin and vinculin between wt and Palld−/− macrophages (Figure S4).
Macrophage protein VCAM-126 and integrin-αV25 are required for macrophages to bind erythroblasts, but we could not detect any significant differences in the expression level or distribution pattern of these 2 proteins in macrophages between wt and mutants (Figure S5). All these data suggest that the specific defect in macrophages in erythroblastic island formation caused by palladin disruption could be due to a novel mechanism or pathway.
In summary, our study demonstrates that palladin, an actin cytoskeleton–associated protein, is crucial for definitive erythropoiesis and erythroblastic island formation, and especially required for normal function of macrophages in fetal liver. Palladin regulates the fetal liver erythropoietic microenvironment that is critical for the survival and differentiation of erythroid cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (39925023), Chinese Ministry of Education (00TPJS111), National Basic Research Program of China (2001CB509901), National High-Tech R&D Program (2001AA216081, 2004AA216080), Science and Technology Commission of Shanghai Municipality (03DJ14088, 05DZ22915), and E-Institutes of Shanghai Municipal Education Commission (E03003, 05BZ13, 072237).
We thank Dr Qiang-Su Guo from the Department of Embryology, Shanghai Jiao Tong University School of Medicine, for technical assistance in confocal microscopy; Dr Jiang Zhu for technical support in hematopoiesis reconstitution; and Prof Bao-Xue Ge, Prof Xiang Gao, and Prof Yong-Rui Zou for their helpful comments and suggestions on this paper.
Authorship
Contribution: X.S.L., X.H.L., Y.W., R.Z.S., Y.E.J., and L.J.Z. performed experiments; L.W., S.Y.L., and H.K. generated and maintained mutant mice; X.S.L., S.J.C., Z.C., J.F., M.M.G., Z.Y.L., and Z.G.W. designed and analyzed experiments; and X.S.L. and Z.G.W. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Zhu-Gang Wang, Laboratory of Genetic Engineering, Department of Medical Genetics, Shanghai Jiao Tong University School of Medicine, 280 South Chong-Qing Road, Shanghai 200025, PR China; e-mail: zhugangw@shsmu.edu.cn.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal