Abstract
Foxp3+ regulatory T cells (Tregs) play a central role in maintaining immune tolerance. A reduction in the function of Tregs is a key feature of autoimmune diseases, whereas their expansion in malignant diseases leads to the suppression of host antitumor responses. We analyzed the absolute number of CD4+ and CD8+ Tregs in the peripheral blood of 52 patients with myelodysplastic syndrome (MDS) and show a significant correlation between increased number of CD4+ Tregs and MDS subgroups with 5% or more bone marrow blasts (P < .001), high International Prognostic Scoring System (IPSS) score (P < .001), and disease progression (P < .001), whereas no correlation between CD8+ Tregs and prognostic variables was observed. The CD4+ Tregs showed a polyclonal spectratype, and the percentage of the naive subset was significantly higher in the high-risk patients compared with low-risk or healthy age-matched donors (P = .032). Our data suggest that CD4+ Treg expansion is a feature of high-risk MDS and progression to aggressive subtypes of the disease.
Introduction
The presence of autoimmune diseases and T-cell–mediated inhibition of hematopoiesis is now a recognized feature of myelodysplastic syndrome (MDS).1,2 Oligoclonal CD8+ T cells occur in up to 95%3 of patients. However, the antigens produced by MDS cells that lead to these T-cell responses are unknown. Recent reports have confirmed that immunosuppressive therapy with antithymocyte globulin and/or cyclosporine A can lead to lasting hematologic responses and abrogation of T-cell clones, which is particularly noticeable in low-risk MDS.4-6 Regulatory T cells (Tregs) play an important role in the immune surveillance of malignancies.7,8 We hypothesized that the effect of Tregs in MDS may be 2-fold: first, expansion of Tregs may inhibit effective immune responses against the dysplastic clone, thereby facilitating disease progression; second, low numbers of Tregs may be associated with low-risk MDS, permitting the emergence of autoreactive T-cell clones and secondary bone marrow hypoplasia
We studied the number of CD4+/CD8+ Tregs and the function and clonality of CD4+ Tregs in the peripheral blood of patients with MDS at different disease stages and correlated the results with known prognostic variables. In order to gain a better understanding of the origin of the expanded Tregs, we analyzed the naive/memory subpopulations in both low- and high-risk MDS.
We demonstrate for the first time a significant increase in the number of CD4+CD25high Foxp3+ Tregs in high-risk disease. The Tregs are polyclonal, and the naive-memory ratio is significantly higher in the high-risk group.
Patients, materials, and methods
Patients
MDS was defined according to the World Health Organization (WHO) classification9 in 52 patients (30 men, 22 women) with a median age of 64.5 years (range,17-83 years). The median age was not different between MDS subgroups (P = .34). All patients were sampled prior to the commencement of any treatment and at least 2 weeks after any blood transfusion. Age-matched controls were obtained from 9 healthy donors. Ethical approval by King's College Hospital Research Ethics Committee (London, United Kingdom) was gained prior to study commencement. Written informed consent was obtained from all patients and controls in accordance with the Declaration of Helsinki.
Mononuclear cell separation
Mononuclear cells were separated from peripheral blood by density gradient sedimentation (Histopaque; Sigma, St Louis, MO). At least 2 × 106 peripheral blood mononuclear cells (PBMCs) were stained for flow cytometry analysis.
Antibodies, reagents, and flow cytometry analysis
PerCP anti-CD3, FITC anti-CD4 monoclonal antibody (mAb), or FITC anti-CD8 (Becton Dickinson, San Jose, CA) and PE anti-CD25 from eBioscience (San Diego, CA) were used for surface antigen staining. PE-Cy5 anti-human Foxp3 (PCH101) and PE-Cy5 rat IgG2a isotype control from eBioscience were used for intracellular Foxp3 staining according to the manufacturer's instructions. The following antibodies were also used for Treg subpopulation analysis: Pacific Blue anti-CD3, FITC anti-CD27, APC anti-CD45RO, and AmCyan anti-CD4 (BD Biosciences, Palo Alto, CA). Flow cytometry was performed by FACSCantoII (Becton Dickinson), and data were analyzed on a BD FACSDiva (Becton Dickinson). Of the CD3+ T cells, the absolute number of CD4+CD25highFoxp3+ and CD4+/CD8+CD25+Foxp3+ cells was calculated. Simultaneous naive and memory subpopulations of CD4+ Tregs were defined by CD25highFoxp3+CD27+CD45RO− and CD25highFoxp3+CD27+CD45RO+, respectively.10,11
Cell sorting and spectratyping
CD4+CD25+ Tregs were sorted using a multistep isolation kit (Miltenyi Biotec, Auburn, CA) designed to isolate CD4+ cells with high expression of CD25. Sorted cells were consistently greater than 90% Foxp3+ (data not shown). Trizol (Invitrogen, Carlsbad, CA) was used for RNA extraction, and first-strand cDNA was generated using the Superscript III kit (Invitrogen). CDR3 of T-cell receptor (TCR) Vβ-chain were amplified using Vβ-specific forward and Cβ reverse primers.12 The CDR3 lengths were analyzed using an ABI 3130xl capillary sequencer (Applied Biosystems, Foster City, CA). The overall complexity of Vβ subfamilies was calculated, and the cloning and sequencing of any skewed spectratype was done as previously described.2,13
Effect of Tregs on responder T cells
Purified responder T cells (CD4+CD25−) from patients with MDS were incubated with anti-CD3/CD28 beads with or without a 1:1 ratio of Tregs. Supernatants were analyzed for concentration of IFN-γ by enzyme-linked immunosorbent assay (ELISA).
Statistical analysis
Statistical analysis was performed using SPSS version 14.0 (Chicago, IL). The nonpaired t test and Mann-Whitney U test were used to compare low- and high-risk groups, and significance was set at a P value less than .05.
Results and discussion
A total of 7 (13%) patients had refractory anemia (RA), 16 (31%) patients had refractory cytopenia with multilineage dysplasia (RCMD), 16 (31%) patients had refractory anemia with excess blasts (RAEB), 9 (17%) patients had 5q− syndrome, and 4 (8%) had myelodysplastic/myeloproliferative disease (MDS/MPD).
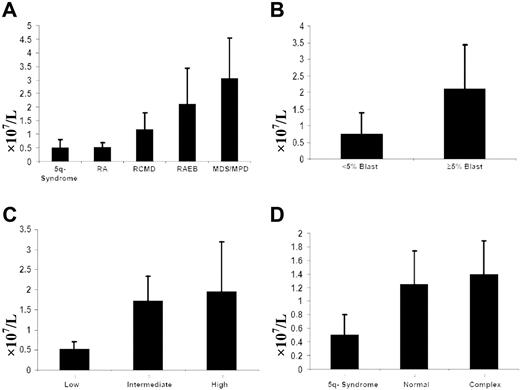
Cytogenetics were normal in 49% of patients, isolated del(5q) was in 17% of patients, complex was in 16% of patients, and stable single abnormalities were present in 18% of patients. The absolute number of CD4+ Tregs was significantly higher in patients with complex cytogenetic abnormalities compared with 5q− syndrome (P = .008; Figure 1D). An International Prognostic Scoring System (IPSS) score of 0 (low risk), 0.5 to 2 (intermediate risk), and 2.5 or higher (high risk) was observed in 18 (35%) of 52 patients, 25 (48%) of 52 patients, and 9 (17%) of 52 of patients, respectively.
The number of CD4+CD25highFoxp3+ Tregs in MDS. (A) The absolute number of Tregs in various subtypes of MDS. The number of Tregs in patients with RAEB was significantly higher compared with low-risk subtypes: 5q− syndrome (P < .001), RA (P < .001), and RCMD (P = .002). (B) The absolute number of Tregs was also significantly higher in patients with 5% or greater bone marrow blasts compared with patients with less than 5% bone marrow blasts (P > .001). (C) Comparison of the absolute number of Tregs from patients with low-risk IPSS (score, 0) to those with intermediate-risk (score, 0.5-2.0) and high-risk IPSS (score, 2.5 or greater). Patients with low-risk MDS demonstrated significantly lower numbers of Tregs than did both intermediate- and high-risk groups (P < .001). (D) In those patients with a complex cytogenetic abnormality, the median number of CD4+ Tregs was significantly higher compared with 5q− syndrome (0.5 × 107/L vs 1.4 × 107/L; P = .008). There was no significant difference in the number of Tregs upon comparison of patients with normal or complex cytogenetics (P = .29). Error bars represent the interquartile (IQR) of the median.
The number of CD4+CD25highFoxp3+ Tregs in MDS. (A) The absolute number of Tregs in various subtypes of MDS. The number of Tregs in patients with RAEB was significantly higher compared with low-risk subtypes: 5q− syndrome (P < .001), RA (P < .001), and RCMD (P = .002). (B) The absolute number of Tregs was also significantly higher in patients with 5% or greater bone marrow blasts compared with patients with less than 5% bone marrow blasts (P > .001). (C) Comparison of the absolute number of Tregs from patients with low-risk IPSS (score, 0) to those with intermediate-risk (score, 0.5-2.0) and high-risk IPSS (score, 2.5 or greater). Patients with low-risk MDS demonstrated significantly lower numbers of Tregs than did both intermediate- and high-risk groups (P < .001). (D) In those patients with a complex cytogenetic abnormality, the median number of CD4+ Tregs was significantly higher compared with 5q− syndrome (0.5 × 107/L vs 1.4 × 107/L; P = .008). There was no significant difference in the number of Tregs upon comparison of patients with normal or complex cytogenetics (P = .29). Error bars represent the interquartile (IQR) of the median.
The median number of CD4+CD25highFoxp3+ Tregs in 5q− syndrome was 0.51 × 107/L (range, 0.2-1.07 × 107/L); in RA, 0.52 × 107/L (range, 0.5-1.29 × 107/L); in RCMD, 1.18 × 107/L (range, 0.24-2.34 × 107/L); in RAEB, 2.11 × 107/L (range, 0.8-7.06 × 107/L); and in MDS/MPD, 3.06 × 107/L (range, 0.8-5.0 × 107/L). Median CD4+ Tregs were significantly higher in patients with 5% or more bone marrow blasts compared with less than 5% BM blasts (2.11 × 107/L vs 0.75 × 107/L; P < .001) and in high IPSS scores compared with low/intermediate IPSS scores (1.96 × 107/L vs 0.51 × 107/L; P < .001) despite no difference in the median age between the 2 groups. No significant correlation was observed between the number of Tregs with platelet count (P = .66) or neutrophil count (P = .07). The number of Tregs in the 14 transfusion-dependent patients was slightly lower than in nondependent patients (0.95 × 107/L vs 1.24 × 107/L), but not statistically significant (P = .67).
Although the number of patients in the MDS/MPD group is small, the mean CD4+ Treg numbers were higher than that of other subgroups. Similarly, patients studied at the time of disease progression (n = 17) had significantly elevated CD4+ Tregs compared with 35 patients with stable disease (2 × 107/L vs 0.69 × 107/L; P < .001; Figure 1). The number of CD4+ Tregs was lower in patients with 5q− syndrome, RCMD, and RA, but was not statistically different from healthy controls (P = .6), whereas patients with RAEB and MDS/MPD had significantly higher CD4+ Tregs than healthy controls (P < .001 and P = .02, respectively). It is notable that among patients with RCMD, 7 had CD4+ Tregs that were in the normal range (0.62% ± 0.78% of CD3+ T cells) and 9 had CD4+ Tregs in the high-risk range (1.42% ± 2.7%), reflecting the biological heterogeneity of this subgroup.14-16 There was no difference in the number of CD8+ Tregs between MDS subtypes (P = .28), IPSS (P = .19), or disease progression (P = .19).
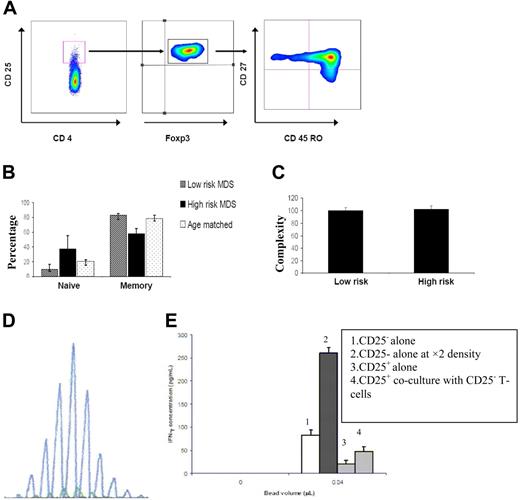
Subpopulation of Treg CD3+CD4+CD25highFoxp3+CD27+CD45RO− and CD3+CD4+CD25highFoxp3+CD27+CD45RO+ subsets were analyzed in 10 patients with the highest and lowest numbers of Tregs and 9 age-matched controls. The percentage of naive Tregs was significantly higher in high-risk patients compared with low-risk and healthy subjects (P = .032; Figure 2A,B). The ratio of naive to memory Tregs was also significantly higher in the high-risk group than in the low-risk (P = .016) or control groups (P = .032).
Spectratype and flow cytometry data demonstrating polyclonality of Tregs and expansion of the naive subset. (A) Flow cytometry data demonstrating the expanded subpopulation of Tregs. CD4+CD25highFoxp3+ cells were labeled with CD27 and CD45RO, and naive and memory subsets of Tregs were identified. CD4+CD25highFoxp3+CD27+CD45RO− Tregs were considered to be naive Tregs, and CD4+CD25highFoxp3+CD27+CD45RO+ were considered to be memory Tregs. (B) The percentage of naive Tregs was significantly higher in high-risk patients compared with low-risk patients and healthy age-matched controls (P = .032), whereas there was no significant difference in the percentage of memory Tregs (P = .28). (C) The complexity level of Tregs determined by spectratyping was not significantly different between the low-risk and high-risk cohorts of patients (P = .54). (D) A sample of the polyclonal spectratype of CD4+CD25highFoxp3+ Tregs. Spectratyping has been done on CD4+CD25high T cells to investigate the clonality of Tregs. The overall complexity of a Vβ subfamily was determined by counting the number of discrete peaks per Vβ subfamily. A score of 5 was given to a spectratype with 5 or more peaks. For the spectratypes with 1 to 4 peaks, a score of 1 to 4 was given, respectively. No spectratype signal was given a score of 0. The maximum complexity score for each patient would be 120 (5 × 24 = 120). (E) Standard sandwich ELISA performed on day 5 of culture. Reduced IFN-γ production by CD4+CD25low cells from a patient with MDS stimulated with anti-CD3/CD28 beads (Dynal, Oslo, Norway) with or without a 1:1 ratio of Tregs to responder cells in 250 μL complete RPMI 1640 culture medium (Invitrogen, Paisley, United Kingdom) containing 2% FCS and supplemented with penicillin, streptomycin, and l-glutamine (PAA Laboratories GmbH, Haidmanweg, Austria). FACS plot generated by FlowTo (Tree Star Inc, Ashland, OR) for publication only.
Spectratype and flow cytometry data demonstrating polyclonality of Tregs and expansion of the naive subset. (A) Flow cytometry data demonstrating the expanded subpopulation of Tregs. CD4+CD25highFoxp3+ cells were labeled with CD27 and CD45RO, and naive and memory subsets of Tregs were identified. CD4+CD25highFoxp3+CD27+CD45RO− Tregs were considered to be naive Tregs, and CD4+CD25highFoxp3+CD27+CD45RO+ were considered to be memory Tregs. (B) The percentage of naive Tregs was significantly higher in high-risk patients compared with low-risk patients and healthy age-matched controls (P = .032), whereas there was no significant difference in the percentage of memory Tregs (P = .28). (C) The complexity level of Tregs determined by spectratyping was not significantly different between the low-risk and high-risk cohorts of patients (P = .54). (D) A sample of the polyclonal spectratype of CD4+CD25highFoxp3+ Tregs. Spectratyping has been done on CD4+CD25high T cells to investigate the clonality of Tregs. The overall complexity of a Vβ subfamily was determined by counting the number of discrete peaks per Vβ subfamily. A score of 5 was given to a spectratype with 5 or more peaks. For the spectratypes with 1 to 4 peaks, a score of 1 to 4 was given, respectively. No spectratype signal was given a score of 0. The maximum complexity score for each patient would be 120 (5 × 24 = 120). (E) Standard sandwich ELISA performed on day 5 of culture. Reduced IFN-γ production by CD4+CD25low cells from a patient with MDS stimulated with anti-CD3/CD28 beads (Dynal, Oslo, Norway) with or without a 1:1 ratio of Tregs to responder cells in 250 μL complete RPMI 1640 culture medium (Invitrogen, Paisley, United Kingdom) containing 2% FCS and supplemented with penicillin, streptomycin, and l-glutamine (PAA Laboratories GmbH, Haidmanweg, Austria). FACS plot generated by FlowTo (Tree Star Inc, Ashland, OR) for publication only.
The clonality of CD4+ Tregs was analyzed by the spectratyping of 6 low-risk and 9 high-risk patients. The spectratype of CD4+CD25+ TCR amplicons showed a polyclonal pattern, and the overall complexity of Vβ spectratypes (confirmed by sequencing) was 100 peaks (range, 77-105 peaks) in the high-IPSS-score group and 102 peaks (range, 75-110 peaks) in the low-IPSS-score group (P = .54; Figure 2C,D). This finding, in addition to increased naive Tregs suggests that in MDS, like in other malignancies, the expanded Tregs are not clonal and may arise by peripheral expansion.8 By contrast, the spectratype of CD8+ T cells in 10 samples (4 low, 3 intermediate, and 3 high IPSS scores) was skewed on average in 6 of 24 Vβ subfamilies.
The suppressive effect of CD4+ Tregs from patients with MDS was demonstrated by a reduced level of IFN-γ in cocultures containing Tregs compared with responder cells alone (Figure 2E).
Our data show that Foxp3+ Treg expansion occurs frequently in high-risk MDS as well as at disease progression. The increase is predominantly in the naive subset, as has been reported previously in other hematologic malignancies, suggesting peripheral expansion.8 By contrast, in low-risk MDS, the Treg population tends to be lower, thereby permitting the emergence of autoimmune responses, including those directed against the dysplastic clone.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the nursing and medical staff in the department of hematological medicine at King's College Hospital for providing clinical samples and Dr Stephen Devereux and Dr Piers Patten for providing healthy control samples. We also thank Professor T. Hamblin and Dr Ziyi Lim for their critical review of the manuscript.
This work was supported by King's College Hospital Joint Research Committee and King's College London. W.I. is funded by the Leukemia Research Fund. M.W.'s other affiliation is Institute of Immunology, Charite Medical School, Berlin, Germany.
Authorship
Contribution: S.K. designed and performed research, analyzed and interpreted data, and drafted the manuscript; W.I. provided clinical data and drafted the manuscript; J.H. provided clinical samples; D.D. designed research; L.B. designed and interpreted data; B.A. performed research and analyzed data; G.L. designed research; M.W. provided research tools; J.M. provided research tools and drafted the manuscript; F.F. designed research, interpreted data, and drafted the manuscript; and G.J.M. designed research, interpreted data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ghulam J. Mufti, Department of Hematological Medicine, Kings College London, Rayne Institute, 123 Coldharbour Lane, London, United Kingdom, SE5 9NU; e-mail: ghulam.mufti@kcl.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal