Abstract
In primary human T cells, anergy induction results in enhanced p59Fyn activity. Because Fyn is the kinase primarily responsible for the phosphorylation of PAG (the phosphoprotein associated with glycosphingolipid-enriched microdomains), which negatively regulates Src-kinase activity by recruiting Csk (the C-terminal Src kinase) to the membrane, we investigated whether anergy induction also affects PAG. Analysis of anergic T cells revealed that PAG is hyperphosphorylated at the Csk binding site, leading to enhanced Csk recruitment and inhibitory tyrosine phosphorylation within Fyn. This together with enhanced phosphorylation of a tyrosine within the SH2 domain of Fyn leads to the formation of a hyperactive conformation, thus explaining the enhanced Fyn kinase activity. In addition, we have also identified the formation of a multiprotein complex containing PAG, Fyn, Sam68, and RasGAP in stimulated T cells. We demonstrate that PAG-Fyn overexpression is sufficient to suppress Ras activation in Jurkat T cells and show that this activity is independent of Csk binding. Thus, in addition to negatively regulating Src family kinases by recruiting Csk, PAG also negatively regulates Ras by recruiting RasGAP to the membrane. Finally, by knocking down PAG, we demonstrate both enhanced Src kinase activity and Ras activation, thereby establishing PAG as an important negative regulator of T-cell activation.
Introduction
In the thymus, most T cells undergo negative selection as they bind with high affinity to the self-peptide/MHC complexes presented. However, thymic selection is not a tight process and some autoreactive T cells escape into the periphery.1 To protect the body from these potentially destructive cells, there must exist mechanisms of peripheral tolerance that prevent autoreactive T cells from becoming activated; one such mechanism is anergy.2
T cells receiving a strong signal via the T-cell receptor (TCR) [signal 1] in the absence of costimulation [signal 2] enter into an unresponsive state (ie, anergy) in which the cells are alive but fail to display functional responses, such as proliferation in response to full activation [signal 1 + 2].3,4 Although the mechanism responsible for the induction of anergy remains unknown, the characterization of anergic T cells has clearly demonstrated that the lack of proliferation is due to a block in interleukin-2 (IL-2) production and that anergic T cells proliferate after addition of exogenous IL-2. The defect in IL-2 production is attributed to a block in Ras activation,5-7 a small G-protein that activates the mitogen-activated protein kinase cascade, ultimately resulting in activation of the transcription factor activator protein-1 (AP-1). Indeed, the ionomycin-induced anergy model shows that activation of nuclear factor of activated T cells in the absence of AP-1 and nuclear factor of the κ-enhancer in B cells (NF-κB) leads to the transcription of genes that induce anergy.8
Within the TCR signaling cascade, the earliest alteration detected in anergic T cells is the up-regulation of the Src family kinase (SFK) p59Fyn.9,10 However the mechanism by which Fyn contributes to anergy is unknown. In addition to the TCR,11 Fyn also binds the phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG)12 (also known as the Csk binding protein [Cbp]).13 Fyn is the kinase responsible for PAG phosphorylation.14 PAG, a transmembrane adaptor that localizes within the lipid rafts, negatively regulates Src kinase activity by recruiting the C-terminal Src kinase (Csk) to the membrane.15 Csk in turn phosphorylates the inhibitory tyrosines conserved within the Src kinases.16 It is noteworthy that T cells overexpressing PAG are resistant to activation via the TCR, a phenotype similar to anergy.12,17 Therefore, we chose to investigate whether anergy induction leads to changes within PAG that might contribute to the unresponsiveness of anergic T cells.
Materials and methods
Approval for these studies was obtained from the Ethics Committee of the Medical Faculty at the Otto-von-Guericke University, Magdeburg, Germany. Informed consent was obtained in accordance with the Declaration of Helsinki.
Antibodies and reagents
Anti-phosphotyrosine (4G10), anti-CD3 (OKT3), and anti-CD28 (248.23.2) hybridoma supernatants were produced in our institute. Phorbol 12-myristate 13-acetate (PMA) and N-dodecyl β-d-maltoside (lauryl maltoside [LM]) were purchased from Calbiochem (San Diego, CA). Digitonin, Igepal (Nonidet P-40 [NP-40]), glutathione-Sepharose, guanosine diphosphate (GDP), and mouse anti-β-actin (AC-15) were from Sigma (St Louis, MO). Human recombinant interleukin 2 (IL-2) was purchased from Tebu-bio (Offenbach, Germany). Mouse anti-Sam68 was obtained from BD Biosciences (San Jose, CA). Rabbit anti-Src (pY215) was purchased from Abcam (Cambridge, United Kingdom), anti-Src (pY418), anti-Src (pY529), and anti-Lck (pY505) from BioSource International (Camarillo, CA), mouse anti-Pan-Ras (Ab-4) from Oncogene Science (Cambridge, MA), mouse anti-RasGAP (B4F8) and rabbit anti-Csk from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Secondary antibodies (goat-anti-mouse-horseradish peroxidase [HRP] and goat-anti-rabbit-HRP) were obtained from Dianova (Hamburg, Germany). Rabbit polyclonal anti-PAG, anti-linker for activation of T cells (LAT), mouse anti-PAG (PAG-C6), anti-Fyn (Fyn-02), anti-Lck (Lck-04), and anti-CD3 (MEM-92) were made in the Prague laboratory. Rabbit anti-Fyn, rabbit anti-Lck, and rabbit anti-Csk (pS364) were kindly provided by Drs A. Veillette (Clinical Research Institute of Montreal, QC), A. Magee (Imperial College Faculty of Medicine, London, United Kingdom), and K. Taskin (University of Oslo, Norway), respectively. Rabbit anti-phospho-PAG (pY317) was made by immunizing rabbits with the peptide KEISAMpYSS.
T-cell purification
Peripheral blood mononuclear cells were isolated by Ficoll gradient (Biochrom, Berlin, Germany) centrifugation of heparinized blood collected from healthy volunteers. T cells were further purified by non-T cell depletion using the Pan T cell isolation kit II (Miltenyi Biotec Inc, Auburn, CA).
Anergy induction
Anergy was induced as described previously18 with the following modification: 24-well, flat-bottomed tissue culture plates were precoated with 10 μg/mL rabbit-anti-mouse immunoglobulin (Dako Denmark A/S, Glostrup, Denmark) in phosphate-buffered saline (PBS) overnight at 4°C. After washing 3 times with PBS, anti-CD3 (OKT3) supernatant diluted to ∼1 μg/mL was immobilized overnight at 4°C. After washing, T cells were inoculated at 6 × 105 cells/mL in 1 mL/well RPMI 1640 medium containing NaHCO3, stable glutamine, 10% heat-inactivated fetal calf serum (PAN-Biotech GmbH, Aidenbach, Germany) and CiproBay 200 (Bayer AG, Wuppertal, Germany). Rescued samples received an additional 10−9 mol/L PMA. Resting cells were cultured without stimulus.
After 3 days of incubation at 37°C and 5% CO2, the cells were collected, transferred into new plates with fresh medium, and rested for 1 additional day. The cells were then harvested, viability was determined by trypan blue staining, and the dead cells were removed by Ficoll centrifugation.
Proliferation assay
To assess the proliferative capacity, cells were restimulated in 96-well round-bottomed tissue culture plates (Costar; Corning Life Sciences, Acton, MA) coated as described above. Cells were plated at 5 × 104 cells/well in triplicate and restimulated with either plate-bound anti-CD3 (OKT3) alone, plate-bound anti-CD3 plus anti-CD28, plate-bound anti-CD3 plus exogenous IL-2 (100 U/well), or PMA (10−9 M) plus ionomycin (0.25 μg/mL).
[3H]Thymidine (0.3 μCi/well; specific activity, 50 Ci/mmol) was added for the last 8 to 10 hours of the 3-day incubation, and the plates were harvested using a PHD cell harvester (Inotech AG, Basel, Switzerland). Thymidine incorporation was measured by liquid scintillation counting.
Immunoprecipitation and Western blotting
Cells (5 × 106) were lysed in ice-cold buffer (500 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 100 mmol/L NaCl, 1% NP-40, 5 mmol/L ethylenediaminetetraacetic acid [EDTA], 1% LM, 1 mmol/L phenylmethylsulfonyl fluoride [PMSF], 1 mmol/L Na3OV4, 50 mmol/L NaF, and 10 mmol/L Na4P2O7) for 30 minutes and then centrifuged for 15 minutes at 13 000 rpm, 4°C. Postnuclear supernatants were heated with 5× reducing sample buffer for 5 minutes at 95°C.
For immunoprecipitation, 20 × 106 cells were resuspended in 500 μL lysis buffer, as described above. An aliquot (10%) was kept as lysate and the rest was incubated with 1 mg/mL BSA, antibody and protein A Sepharose for 2 to 18 hours with rotation at 4°C. Beads were washed 5 times with low-detergent washing buffer (0.05% NP-40, 5 mmol/L EDTA, 150 mmol/L NaCl, and 50 mmol/L Tris, pH 7.4) and the immunoprecipitated material released in reducing sample buffer. Lysates and immunoprecipitates were separated on 10% SDS-PAGE and analyzed by Western blotting.
Subcellular fractionation
Cells (10 × 106) were lysed in 50 μL buffer I (10 mmol/L HEPES, pH 7.9, 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.1 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid [EGTA], 1 mmol/L dithiothreitol [DTT], 1 mmol/L PMSF, 2 mmol/L Na3OV4, 2 mmol/L NaF, and 10 mmol/L Na4P2O7) for 20 minutes on ice. Then 3 μL of 10% NP-40 was added and incubated for an additional 10 minutes. Samples were then centrifuged at 2000 rpm, 5 minutes, 4°C. Supernatant represents the cytosolic fraction. The pellet was washed twice with buffer I, lysed in 25 μL of buffer II (1% NP-40, 1% LM, 50 mmol/L Tris, pH 7.4, 170 mmol/L NaCl, 1 mmol/L DTT, 1 mmol/L PMSF, 2 mmol/L Na3OV4, 2 mmol/L NaF, and 10 mmol/L Na4P2O7) for 1 hour at 4°C with agitation, and then centrifuged at 13 000 rpm for 10 minutes, 4°C. The supernatant represents the nuclear fraction.
In vitro kinase assay
Cells (10 × 106/sample) were lysed and immunoprecipitated as described above with either PAG-C6 Sepharose; Fyn-02 or Lck-04 and protein A-Sepharose. In vitro kinase assays were performed as described previously.12
cAMP measurement
Cyclic AMP levels from 1 × 105 T cells were determined using the cAMP Biotrak Enzymeimmunoassay System (GE Healthcare, Chalfont St Giles, Buckinghamshire, United Kingdom) according to the manufacturer's instructions. Data were analyzed using Prism software (ver. 3.0; GraphPad Software, San Diego, CA).
Constructs
The vectors pEF-BOS, Fyn, FPAG, and the YΔF mutants and transfection of Jurkat T cells were described previously.12 Additional mutants were generated using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. For siRNA, the human sequence 5′-GCGAUACAGACUCUCAACATT-3′ corresponding to Shima et al19 was either used as RNA oligonucleotides (Invitrogen, Carlsbad, CA) or cloned as shRNA into the vector pCMS3-enhanced green fluorescent protein (EGFP). All constructs were sequenced to ensure integrity. Primary human T cells were transfected using the Nucleofectin kit (Amaxa Biosystems, Gaithersburg, MD) according to the manufacturer's instructions.
Ras activation assay
Cells (18 × 106) were either unstimulated or stimulated with anti-CD3 (OKT3)/anti-CD28 supernatants (1:1) for 1 to 30 minutes at 37°C and lysed in 25 mmol/L HEPES, 150 mmol/L NaCl, 1% NP-40, 10 mmol/L MgCl2, 1 mmol/L EDTA, 1 mmol/L PMSF, 1 mmol/L Na3OV4, 50 mmol/L NaF, and 10 mmol/L Na4P2O7 supplemented with 1 mmol/L GDP, vortexed, and centrifuged at 13 000 rpm, 5 minutes, 4°C. An aliquot (10%) of the lysate served as the loading control. Active Ras was precipitated with Raf1-Ras binding domain (GST-Raf1-RBD) coupled to glutathione-Sepharose by rotating for 45 minutes at 4°C. GST pull-downs were washed twice with lysis buffer (without GDP), heated in 2× reducing sample buffer at 95°C for 5 minutes, separated on 14% SDS-PAGE, blotted and stained with anti-Pan-Ras antibody to detect active Ras.
Quantification
Films were scanned with an Epson Perfection 4990 Photo scanner (Epson America, Torrance, CA) and the optical density determined using Kodak 1D 3.6 software (Eastman Kodak, Rochester, NY). The fold induction (FI) was then calculated as the density of the band of interest in proportion to the density of the loading control normalized to the value in resting cells: FI = [(Fyn/Actin)/(Fynrest/Actinrest)].
Results
Human peripheral blood T cells (purity > 95%) were either untreated (resting), cultured on anti-CD3 coated plates (anergized), or cultured on anti-CD3 coated plates plus PMA to mimic costimulation (rescued). During the 3 days of culture, the cells receiving stimulus proliferated and up-regulated the activation markers CD25 and CD69 as expected (data not shown). Figure 1A shows that after one additional day of resting, the cells were indeed rested, because they no longer proliferate without stimulus. On restimulation with either anti-CD3 or the combination of anti-CD3 plus anti-CD28, both the resting and rescued cells proliferated, whereas anergized cells did not. However, on the addition of exogenous interleukin-2 (IL-2) anergized cells proliferated, showing that they are indeed anergic.
Anergic T cells proliferate only in response to exogenous IL-2 and show markedly enhanced Fyn kinase activity. (A) Peripheral T cells were pretreated with no stimulus (resting), anti-CD3 antibody (anergic), or anti-CD3 antibody plus phorbol ester PMA (rescued) for 3 days. After 1 day of resting, the cells received either no stimulus or were restimulated with anti-CD3, anti-CD3 + anti-CD28, or anti-CD3 + IL-2. Incorporation of [3H]thymidine was measured at 72 hours and is shown as the mean value of triplicates (± SD). Data were analyzed using one-way ANOVA (∗∗, P < .01; ∗∗∗, P < .001). Fyn (B) and Lck (C) were immunoprecipitated from resting (rest.), anergic (aner.), and rescued (resc.) T cells and in vitro kinase assays (IVK) were performed. Phosphorylation was visualized by autoradiography (upper panel), the amount of kinase precipitated was determined by immunoblotting with specific antibodies (lower panel). Phosphorylation of the exogenous substrate enolase and Fyn autophosphorylation were normalized with respect to the level of kinase. These values are presented here as the fold-induction (FI). (D) Expression of Fyn and Lck from total lysate. Actin shows equal loading. (E) Resting, anergic and rescued cells were either left untreated (−) or stimulated via CD3 (MEM-92) for 2 minutes (+), lysed, and subjected to Western blotting. Changes in protein tyrosine phosphorylation are shown. Equal loading is shown with actin staining. (F) To better visualize the defects in proximal signaling, the TCR ζ chain and LAT were immunoprecipitated. Western blots of anti-phosphotyrosine staining and total protein are presented.
Anergic T cells proliferate only in response to exogenous IL-2 and show markedly enhanced Fyn kinase activity. (A) Peripheral T cells were pretreated with no stimulus (resting), anti-CD3 antibody (anergic), or anti-CD3 antibody plus phorbol ester PMA (rescued) for 3 days. After 1 day of resting, the cells received either no stimulus or were restimulated with anti-CD3, anti-CD3 + anti-CD28, or anti-CD3 + IL-2. Incorporation of [3H]thymidine was measured at 72 hours and is shown as the mean value of triplicates (± SD). Data were analyzed using one-way ANOVA (∗∗, P < .01; ∗∗∗, P < .001). Fyn (B) and Lck (C) were immunoprecipitated from resting (rest.), anergic (aner.), and rescued (resc.) T cells and in vitro kinase assays (IVK) were performed. Phosphorylation was visualized by autoradiography (upper panel), the amount of kinase precipitated was determined by immunoblotting with specific antibodies (lower panel). Phosphorylation of the exogenous substrate enolase and Fyn autophosphorylation were normalized with respect to the level of kinase. These values are presented here as the fold-induction (FI). (D) Expression of Fyn and Lck from total lysate. Actin shows equal loading. (E) Resting, anergic and rescued cells were either left untreated (−) or stimulated via CD3 (MEM-92) for 2 minutes (+), lysed, and subjected to Western blotting. Changes in protein tyrosine phosphorylation are shown. Equal loading is shown with actin staining. (F) To better visualize the defects in proximal signaling, the TCR ζ chain and LAT were immunoprecipitated. Western blots of anti-phosphotyrosine staining and total protein are presented.
It has been reported previously that anergized T cells up-regulate Fyn kinase activity.9,10 Analysis of our anergized cells (Figure 1B) showed that Fyn activity was indeed dramatically up-regulated, whereas Lck activity was only slightly increased (Figure 1C). In agreement with these reports, we also observed a specific increase in the protein level of Fyn in anergic T cells (Figure 1D) (1:2.5:1.6), but only a marginal increase in Lck (1:1.7:1.7), similar to rescued cells.
In addition to increased kinase activity, there was also a noticeable change in the phosphotyrosine signature (Figure 1E,F). In resting T cells, only PAG, the Src kinases and the basal phosphorylation of the ζ chain were observed. However, on stimulation, a number of bands were induced. Anergic and rescued cells showed a dramatic increase in phosphorylation in the 50- to 70-kDa range, as well as the induction of a number of bands of 30 kDa or less. In addition, the response to stimulation was also altered, most notably the reduced phosphorylation of ζ and LAT (Figure 1F). Although the increase in tyrosine phosphorylation was in clear agreement with the increased Src kinase activity, the lack of ζ and LAT phosphorylation was not. Note that because both anergic and rescued cells receive the same stimulus via the TCR, the pattern of anti-phosphotyrosine staining is similar. The difference is that rescued cells received PMA, which mimics diacylglycerol (DAG) and thus acts directly on Ras guanyl releasing protein (RasGRP) and protein kinase C-θ (PKC-θ) to promote transcription, thereby preventing anergy induction (Figure 1A).
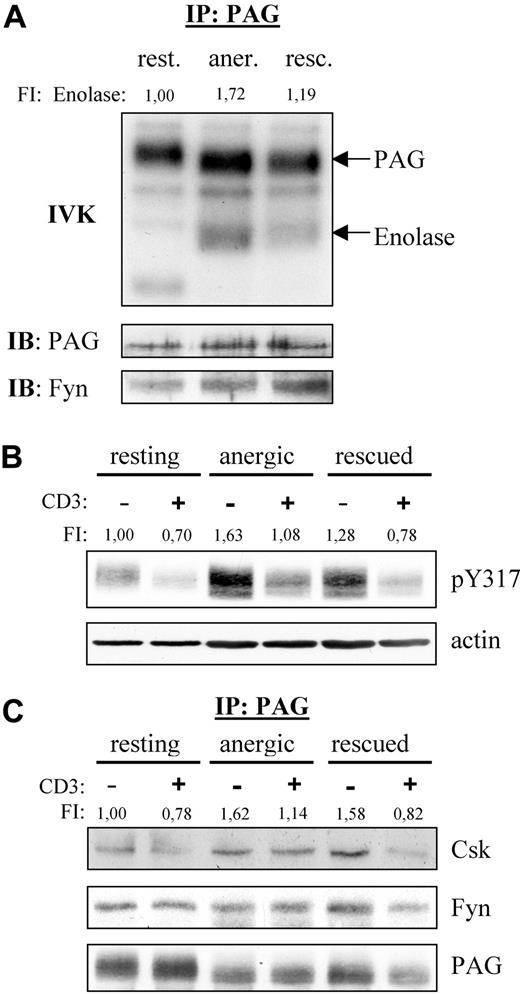
Because Fyn constitutively associates to PAG, we also analyzed the PAG-associated Fyn kinase activity (Figure 2A). We were surprised to find that resting T cells showed no phosphorylation of enolase even though PAG became phosphorylated, indicating that Fyn was indeed active. This suggests that PAG was the preferred substrate. However, in anergized cells, we observed a marked increase in both PAG and enolase phosphorylation compared with rescued cells, clearly demonstrating an increase in PAG-associated Fyn kinase activity in anergic T cells.
PAG is hyperphosphorylated in anergic T cells. (A) Anergic cells showed increased PAG-associated Fyn-kinase activity. PAG was immunoprecipitated from resting (rest.), anergic (aner.), and rescued (resc.) cells, and the PAG-associated kinase activity was measured by IVK. Phosphorylation was visualized by autoradiography (top panel). The amount of precipitated PAG and coprecipitated Fyn were detected by Western blotting (middle and bottom panels). Bands corresponding to PAG and enolase phosphorylation are marked. Phosphorylation of enolase was normalized with respect to the PAG level and is presented here as fold induction. (B) PAG was hyperphosphorylated in anergic cells. Resting, anergic, and rescued T cells were either left untreated (−) or stimulated via CD3 (MEM-92) for 2 minutes (+). Samples were then lysed, subjected to Western blotting, and probed with a phosphospecific antibody recognizing pY317 of PAG (top panel). Actin staining is shown for equal loading (bottom panel). The level of Y317 phosphorylation normalized to actin is presented as the fold-induction. (C) Hyperphosphorylated PAG recruits more Csk in anergic cells. Resting, anergic, and rescued cells were prepared as in panel B. PAG was immunoprecipitated and the associated proteins detected by blotting with anti-Csk and anti-Fyn antibody. The amount of Csk coprecipitated with PAG was normalized to the PAG level and is presented as fold induction.
PAG is hyperphosphorylated in anergic T cells. (A) Anergic cells showed increased PAG-associated Fyn-kinase activity. PAG was immunoprecipitated from resting (rest.), anergic (aner.), and rescued (resc.) cells, and the PAG-associated kinase activity was measured by IVK. Phosphorylation was visualized by autoradiography (top panel). The amount of precipitated PAG and coprecipitated Fyn were detected by Western blotting (middle and bottom panels). Bands corresponding to PAG and enolase phosphorylation are marked. Phosphorylation of enolase was normalized with respect to the PAG level and is presented here as fold induction. (B) PAG was hyperphosphorylated in anergic cells. Resting, anergic, and rescued T cells were either left untreated (−) or stimulated via CD3 (MEM-92) for 2 minutes (+). Samples were then lysed, subjected to Western blotting, and probed with a phosphospecific antibody recognizing pY317 of PAG (top panel). Actin staining is shown for equal loading (bottom panel). The level of Y317 phosphorylation normalized to actin is presented as the fold-induction. (C) Hyperphosphorylated PAG recruits more Csk in anergic cells. Resting, anergic, and rescued cells were prepared as in panel B. PAG was immunoprecipitated and the associated proteins detected by blotting with anti-Csk and anti-Fyn antibody. The amount of Csk coprecipitated with PAG was normalized to the PAG level and is presented as fold induction.
Because Fyn is responsible for PAG phosphorylation and we observed increased PAG-associated Fyn kinase activity, we next investigated whether PAG phosphorylation was altered in anergic T cells (Figure 2B). Western blotting with a phosphospecific reagent to the Csk-binding site (Y317) clearly demonstrated that PAG was hyperphosphorylated in anergic T cells (Figure 2B). In resting T cells, PAG is phosphorylated and, on stimulation, becomes dephosphorylated by an unknown phosphatase. In anergic T cells, the level of PAG phosphorylation also decreased on stimulation, indicating that the phosphatase was still active. An increase in PAG phosphorylation was also observed in the rescued control, but this increase was not as dramatic as that observed in anergic cells. In addition, the dephosphorylation observed in rescued cells was comparable with that in resting T cells. It is noteworthy that the level of PAG phosphorylation observed in restimulated anergic T cells never fell below that seen in resting unstimulated cells. As expected, the level of PAG-associated Csk is also increased, whereas the level of PAG-associated Fyn remained unchanged (Figure 2C).
Because Csk phosphorylates the inhibitory tyrosine residues conserved within the C termini of the Src-kinases, we next investigated whether the increased Csk-association also reflected a change in the phosphorylation status of these residues. Indeed, Y529 phosphorylation of Fyn was increased by 100% in anergic T cells with respect to resting T cells (Figure 3A). Although the level of inhibitory tyrosine phosphorylation was also increased in rescued cells (+30%), the change was not nearly as dramatic. Because both populations received the same signal via the TCR, this difference must reflect changes due to PMA. Further differences become apparent when one examines phosphorylation of the activatory tyrosines. We were surprised to find that despite the increased kinase activity observed in Figure 2, both Fyn and Lck showed a decreased phosphorylation of their activatory tyrosines, Y418 and Y394, respectively, in both anergic and rescued cells (Figure 3A,B), thus suggesting that autophosphorylation is not a direct measure of kinase activity. In contrast to Fyn, the inhibitory residue within Lck (Y505) was not altered in anergic cells, because little Lck resides within the lipid rafts.20
Fyn tyrosine phosphorylation at Y215 and Y529 is markedly increased in anergic T cells. (A) Lysates of resting, anergic, and rescued T cells were probed with phosphospecific antibodies for either the activatory or the inhibitory tyrosine of Fyn, Y418 and Y529, respectively. The level of tyrosine phosphorylation was normalized to total Fyn and is presented as the fold-induction. (B) The samples in panel A were probed with phosphospecific antibodies against the activatory and inhibitory tyrosines of Lck, Y394, and Y505 and normalized to total Lck. Black vertical lines have been inserted to indicate where these images were cut together from one film. (C) Cyclic AMP (cAMP) levels were determined. The data represent the mean (± SD) from 3 independent experiments. Data were analyzed using one-way ANOVA (∗∗, P < .01). (D) The level of Ser364 phosphorylation within Csk was determined by Western blotting. Total Csk staining is also shown. The level of phosphorylation normalized to total Csk is presented as the fold induction. (E) To investigate the mechanism responsible for increased Fyn kinase activity, Y215 phosphorylation was investigated. Anti-pY215 immunoprecipitates from resting, anergic, and rescued T cells were probed with anti-Fyn (top), anti-pY529 (middle), and anti-Lck (bottom). Because the IPs from anergic T cells contained more Fyn, we therefore concluded that phosphorylation of this site was enhanced in anergic cells.
Fyn tyrosine phosphorylation at Y215 and Y529 is markedly increased in anergic T cells. (A) Lysates of resting, anergic, and rescued T cells were probed with phosphospecific antibodies for either the activatory or the inhibitory tyrosine of Fyn, Y418 and Y529, respectively. The level of tyrosine phosphorylation was normalized to total Fyn and is presented as the fold-induction. (B) The samples in panel A were probed with phosphospecific antibodies against the activatory and inhibitory tyrosines of Lck, Y394, and Y505 and normalized to total Lck. Black vertical lines have been inserted to indicate where these images were cut together from one film. (C) Cyclic AMP (cAMP) levels were determined. The data represent the mean (± SD) from 3 independent experiments. Data were analyzed using one-way ANOVA (∗∗, P < .01). (D) The level of Ser364 phosphorylation within Csk was determined by Western blotting. Total Csk staining is also shown. The level of phosphorylation normalized to total Csk is presented as the fold induction. (E) To investigate the mechanism responsible for increased Fyn kinase activity, Y215 phosphorylation was investigated. Anti-pY215 immunoprecipitates from resting, anergic, and rescued T cells were probed with anti-Fyn (top), anti-pY529 (middle), and anti-Lck (bottom). Because the IPs from anergic T cells contained more Fyn, we therefore concluded that phosphorylation of this site was enhanced in anergic cells.
Several factors may explain the increase in inhibitory tyrosine phosphorylation in anergic T cells; one is increased Csk activity and another is decreased phosphatase activity. First, the kinase activity of Csk is increased on binding to PAG.21 Therefore, the increase is certainly due in part to the hyperphosphorylation of PAG and increased Csk binding compared with resting cells (Figure 2B,C); however, this alone was not entirely sufficient, because the PAG-associated Csk levels were relatively equal between the anergized and rescued T cells, whereas inhibitory tyrosine phosphorylation was not. Second, the phosphorylation of Csk at Ser364 by cAMP-dependent protein kinase (PKA) also increases Csk activity, which is additive to the increase observed with PAG binding.22 Because the levels of cAMP increase on TCR stimulation,23 we measured the cAMP levels within our cells (Figure 3C). Indeed, we found that both anergic and rescued T cells had an approximate 2.5-fold increase compared with resting cells. Although the increase in cAMP levels was similar, anergic T cells showed a 20% increase in the PKA-dependent phosphorylation at Ser364 of Csk, whereas rescued cells show a comparable decrease (Figure 3D). Although several factors argue for increased Csk activity, they do not seem to be proportional; therefore, we conclude that the difference in inhibitory tyrosine phosphorylation must also represent changes in the phosphatase activity of either CD45 or receptor protein tyrosine phosphatase α (RPTPα).
That the increase in Fyn kinase activity coincides with increased inhibitory tyrosine phosphorylation may seem puzzling at first, in that inhibitory tyrosine phosphorylation is often associated with a closed-inactive conformation.24 However, disruption of the SH3-linker interaction is believed to be the first step in activating Src-kinases.25 In our case, the constitutive interaction between Fyn and PAG is proposed to occur via the SH3 domain, because Fyn binding is phospho-independent,12 and occupation of the SH3 domain increases Fyn kinase activity.26 In addition, other sites of phosphorylation exist that regulate Src kinase activity.25 One such site (Y215) within the SH2 domain enhances kinase activity particularly in combination with inhibitory tyrosine phosphorylation. Because this residue is conserved among SFKs, it is difficult to determine by Western blotting whether the increase in signal is specific for Fyn (data not shown). Therefore, we immunoprecipitated this species and probed for Fyn. One can clearly see that Y215 phosphorylation in Fyn increased within anergic T cells (Figure 3E). Although the increased phosphorylation at both Y215 and Y529 might explain the increase in Fyn kinase activity observed in anergic T cells, it does not explain how Fyn contributes to anergy or why proximal signaling events (ie, ζ-phosphorylation) are defective in anergic T cells. The latter seems similar to the block in activation observed in cells overexpressing PAG,12 which is clearly mediated by Csk binding.17 Because we observed PAG hyperphosphorylation, increased Csk recruitment, and inhibitory tyrosine phosphorylation of Fyn, it seems that PAG might contribute to the defect in proximal signaling.
Enhanced Fyn kinase activity predisposes T cells to calcium signaling,27 and anergic T cells possess increased cytosolic calcium.28 The ionomycin-induced anergy model demonstrates that calcium signaling alone is sufficient to induce anergy. However, on a molecular level, anergy (or at least the defect in IL-2 production) results from a block in Ras activation. Thus, it is not clear how signals from the TCR should initiate a calcium response without activating other pathways, such as Ras or PKC-θ, which in turn activate AP-1 and NF-κB, particularly because activated phospholipase Cγ1 (PLCγ1), which is required for calcium signaling, generates both inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Although IP3 triggers intracellular calcium release, DAG activates both RasGRP and PKCθ. One enzyme that could bias this pathway to favor calcium signaling is diacylglycerol kinase (DGK), which converts DAG to phosphatidic acid.29 However, we found no change in the expression of either isoform that would suggest a contribution of DGK to anergy (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article), which is contrary to recent reports30,31 ; one possible explanation is that these studies were performed in mouse, whereas ours was done in human.
Ras are a family of small GTPases that localize to membranes on S-acylation of a CAAX motif within their C-terminus. Ras activity is regulated via cycling between a GDP- (inactive) and a GTP-bound state (active). The exchange of GDP for GTP is mediated by guanine-nucleotide exchange factors (GEFs), such as RasGRP and SOS (son of sevenless). The intrinsic GTPase activity of Ras returns it to the inactive state and can be enhanced by association with GTPase-activating proteins (GAPs), such as RasGAP.
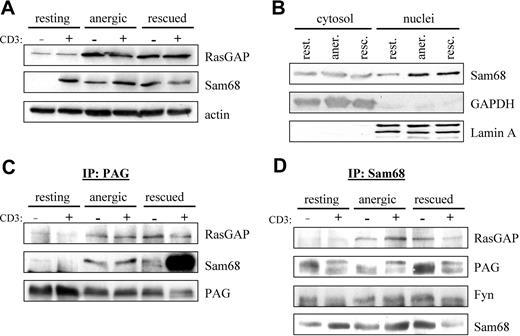
Because we observed enhanced Fyn kinase activity (Figure 2A) as well as enhanced phosphorylation of proteins in the 50- to 70-kDa range in anergized T cells (Figure 2D), we searched the literature for proteins that were substrates of Fyn that could also interact with RasGAP. Two likely candidates were the cytosolic adaptor protein p62dok (dowstream of tyrosine kinase 1 [Dok1])32,33 and the nuclear/cytosolic protein Sam68 (Src-associated in mitosis 68 kDa, also known as KH domain containing, RNA binding, signal transduction associated 1 [KHDRBS1]).34 We first checked whether the expression of these proteins was altered by anergy induction. Although probing of postnuclear cell lysates showed no change in p62dok expression (data not shown), we did observe a clear increase in RasGAP expression (Figure 4A), indicating that this gene was induced on activation. Because the nuclei were not present, it is difficult to say whether there was an increase in total Sam68 expression; however, the localization of Sam68 within the cell seems altered. A second fractionation protocol (Figure 4B) shows a clear increase in nuclear Sam68, whereas the cytosolic fraction remains constant. Taken together, these results suggest that the expression of total Sam68 has also increased.
PAG formed a novel multiprotein complex with Fyn, Sam68, and RasGAP. (A) Increased Sam68 and RasGAP expression. Resting, anergic and rescued cells were untreated (−) or stimulated with MEM-92 for 2 minutes (+) before the lysis. Postnuclear lysates were blotted with specific antibodies against Sam68 and RasGAP. Actin staining is shown for equal loading. (B) To better determine whether Sam68 expression or localization was altered, cytoplasmic and nuclear fractions were prepared. GAPDH and Lamin A are presented as markers. (C) Sam68 and RasGAP associate with PAG. Cells were prepared as in panel A. PAG was then immunoprecipitated and associated proteins were detected by blotting with anti-RasGAP and anti-Sam68 antibody. The amount of precipitated material is shown with anti-PAG staining. (D) PAG, Fyn, and RasGAP association with Sam68. Samples prepared as in panel A were used to immunoprecipitate Sam68 and then probed with anti-RasGAP, anti-PAG, and anti-Fyn antibodies. The amount of precipitated material is shown by probing with anti-Sam68 antibody.
PAG formed a novel multiprotein complex with Fyn, Sam68, and RasGAP. (A) Increased Sam68 and RasGAP expression. Resting, anergic and rescued cells were untreated (−) or stimulated with MEM-92 for 2 minutes (+) before the lysis. Postnuclear lysates were blotted with specific antibodies against Sam68 and RasGAP. Actin staining is shown for equal loading. (B) To better determine whether Sam68 expression or localization was altered, cytoplasmic and nuclear fractions were prepared. GAPDH and Lamin A are presented as markers. (C) Sam68 and RasGAP associate with PAG. Cells were prepared as in panel A. PAG was then immunoprecipitated and associated proteins were detected by blotting with anti-RasGAP and anti-Sam68 antibody. The amount of precipitated material is shown with anti-PAG staining. (D) PAG, Fyn, and RasGAP association with Sam68. Samples prepared as in panel A were used to immunoprecipitate Sam68 and then probed with anti-RasGAP, anti-PAG, and anti-Fyn antibodies. The amount of precipitated material is shown by probing with anti-Sam68 antibody.
Because RasGAP was suggested to bind directly to PAG,12,35 we next performed PAG immunoprecipitations (IPs) to determine whether we could detect RasGAP association in vivo. Figure 4C shows that we detect weak RasGAP association in resting T cells that increases on prolonged stimulation similar to the protein level (Figure 4A). Because Sam68 binds to RasGAP,34 we also probed PAG IPs for Sam68 association. Similar to RasGAP, we observe weak Sam68 association in resting T cells, that becomes clearly visible in both anergized and rescued cells (Figure 4C). The abundant association of Sam68 in rescued cells is due to the presence of PMA, in that a recent report36 demonstrates that Ser/Thr phosphorylation influences the translocation of Sam68; thus it seems that PMA treatment primes Sam68 for nuclear export. To confirm the specificity of these interactions, we also performed the reciprocal IPs (Figure 4D). It is noteworthy that in resting T cells, the cytoplasmic pool of Sam68 seems to be constitutively associated with PAG and Fyn, but not RasGAP. Therefore, we hypothesize that Sam68 binds to PAG via Fyn, because Sam68 possesses no known protein interaction domains, but does possess binding motifs for both SH2 and SH3 domains; further investigation is required to define this interaction.
That we can better detect PAG in Sam68 IPs rather than Sam68 in PAG IPs may reflect differences in the ability of the respective antibodies to recognize the complex; however, that we can clearly isolate the complex when the level of expression increases rather suggests that we are at the level of detectability in resting cells. An additional explanation may be that complex stability increases with Fyn kinase activity (Figures 2–3) because these interactions are mediated by SH2 domains. Together, our results demonstrate the existence of a multimolecular complex containing PAG, Fyn, Sam68, and RasGAP. Because PAG is present within lipid rafts, this complex would be ideally positioned to block Ras activation, because Ras-GDP is itself raft-localized.37 The enhanced recruitment of RasGAP to this membrane compartment would stimulate the rapid hydrolysis of any Ras-GTP that forms before it had the possibility to translocate.
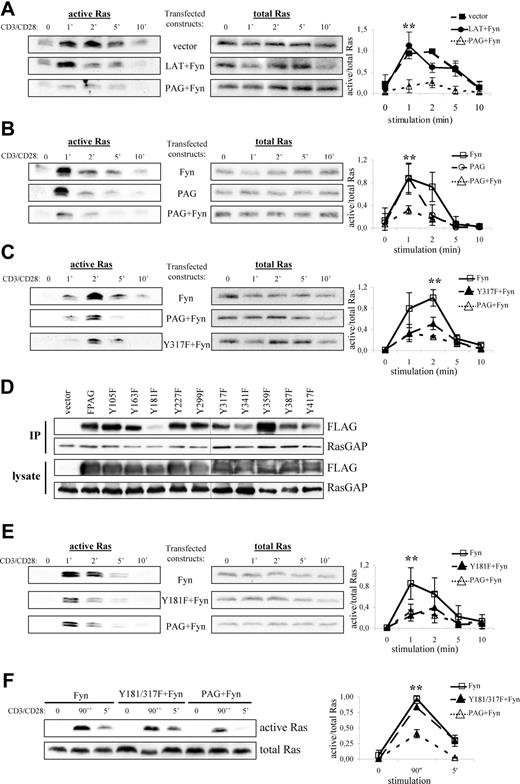
To test this hypothesis, we transfected PAG and Fyn into Jurkat T cells, thereby attempting to mimic the conditions observed in anergic T cells, and measured Ras activation on TCR stimulation. As shown in Figure 5A, cells transfected with vector alone activate Ras with normal kinetics,38 whereas cells overexpressing LAT plus Fyn demonstrated a more transient Ras activation compared with vector alone. This is not surprising because LAT recruits both PLCγ1 and Grb2-SOS, which contribute to Ras activation. Remarkably, cells overexpressing PAG plus Fyn strongly suppressed Ras activation, which was not observed in cells expressing Fyn alone (Figure 5B). Finally, cells expressing PAG alone showed a more truncated response, although the peak of Ras activation was comparable with that in cells expressing Fyn alone. Interestingly, despite expressing similar levels of PAG, we found that the inability to activate Ras seems to correspond with the level of PAG phosphorylation, as indicated by pY317 staining (Figure S2).
PAG overexpression and hyperphosphorylation lead to the block in Ras activation. Jurkat T cells were transfected with constructs encoding for either PAG, Y317F, Fyn, LAT, and/or vector alone as indicated. The following day, cells were stimulated with anti-CD3 and anti-CD28 for the indicated times, lysed, and active Ras (ie Ras-GTP) isolated using the Raf1-RBD (A-C, E, and F, left panels). Total Ras is shown to prove that equal amounts of material were used for this assay (A-C, E, and F, right panels). Representative experiments are shown. A graphical representation of the data is also presented. Values represent the mean (± SD) of at least 3 independent experiments. Data were analyzed using one-way ANOVA (∗, P < .05). Changes in protein expression and phosphorylation after transfection are presented using immunoblots from lysates probed with the indicated antibodies (Figure S2). (D) Jurkat T cells were transfected with constructs encoding for either PAG, the individual YΔF mutants, or vector alone. RasGAP was then immunoprecipitated and the blots probed for associated PAG (anti-FLAG) staining and RasGAP. Blots of the transfected cell lysates are also shown. A black line has been inserted to indicate that, due to space limitations, the samples were resolved on 2 gels.
PAG overexpression and hyperphosphorylation lead to the block in Ras activation. Jurkat T cells were transfected with constructs encoding for either PAG, Y317F, Fyn, LAT, and/or vector alone as indicated. The following day, cells were stimulated with anti-CD3 and anti-CD28 for the indicated times, lysed, and active Ras (ie Ras-GTP) isolated using the Raf1-RBD (A-C, E, and F, left panels). Total Ras is shown to prove that equal amounts of material were used for this assay (A-C, E, and F, right panels). Representative experiments are shown. A graphical representation of the data is also presented. Values represent the mean (± SD) of at least 3 independent experiments. Data were analyzed using one-way ANOVA (∗, P < .05). Changes in protein expression and phosphorylation after transfection are presented using immunoblots from lysates probed with the indicated antibodies (Figure S2). (D) Jurkat T cells were transfected with constructs encoding for either PAG, the individual YΔF mutants, or vector alone. RasGAP was then immunoprecipitated and the blots probed for associated PAG (anti-FLAG) staining and RasGAP. Blots of the transfected cell lysates are also shown. A black line has been inserted to indicate that, due to space limitations, the samples were resolved on 2 gels.
One obvious explanation is that by recruiting Csk, PAG suppresses Src kinase activation and thereby prevents subsequent signaling events, such as ZAP-70 activation and LAT phosphorylation, which are required for Ras activation. To exclude this possibility, we transfected PAG molecules lacking the Csk binding site (Y317F)12 and analyzed Ras activation. As seen in Figure 5C, both wild-type PAG and the Y317F mutant were capable of suppressing Ras activation to nearly the same extent, thereby excluding Csk from sole responsibility for the suppressive effect. To demonstrate that both molecules function as expected, we analyzed the inhibitory tyrosine phosphorylation. Only cells expressing wild-type PAG showed a strong increase in inhibitory tyrosine phosphorylation indicative of increased Csk recruitment to the membrane (Figure S3A). In addition, only wild-type PAG recruited Csk (Figure S3B), whereas both molecules bound RasGAP (Figure S3C), thus demonstrating that Csk and Y317 were not required for RasGAP association; that PAG-RasGAP association increased on pervanadate stimulation indicated a phospho-dependent interaction, (eg, SH2 domain binding) (data not shown). Therefore, to map the RasGAP binding site, we used the PAG-YΔF mutants.12 Immunoprecipitation of RasGAP from PAG-YΔF-transfected Jurkat T cells revealed that Y181 constitutes the RasGAP binding site within PAG (Figure 5D). However, functional analysis of the Y181F mutant showed that it was still capable of suppressing Ras activation (Figure 5E), which we postulated was due to Csk binding. Analysis of the double mutant (Figure 5F) showed that this is indeed the case and that mutation of both binding sites ablates the ability of PAG to suppress Ras activation.
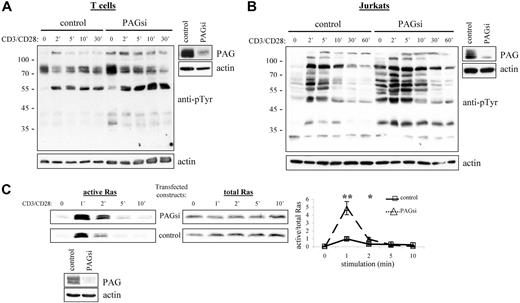
From our results, we would then conclude that PAG is an important negative regulator of cellular activation. Thus, it was quite unexpected that PAG-deficient mice should seem perfectly normal39,40 (J.A.L., unpublished observations, September 2004 to September 2005). Further investigation of these mice has, however, revealed the development of a compensatory mechanism to regulate Src kinase activity (Sabine Lindquist, manuscript in preparation). Thus, to determine the role of PAG, we turned to RNA interference (Figure 6A,B). Suppression of PAG expression in T cells clearly showed that basal Src kinase activity was indeed enhanced and that, on stimulation, activation was also prolonged. In addition, we could show that this leads to a dramatic enhancement in Ras activation (Figure 6C). Together, these results clearly indicated that PAG is indeed an important negative regulator.
PAG down-regulation enhanced both Src kinase activity and Ras activation. Primary human T cells (A) and Jurkat T cells (B) were transfected with siRNA oligonucleotides or the plasmid pCMS3-EGFP containing PAG shRNA, respectively. The down-regulation of PAG protein expression compared with the control is shown. Cells were stimulated with CD3 and CD28 for the times indicated, and lysates blotted with anti-phosphotyrosine. Actin staining is included to show equal loading. (C) In addition, the ability of transfected Jurkat cells to activate Ras was determined using the GST-Raf1-RBG pull-down assay. Total Ras is included as the control and the normalization of active/total Ras appears in the graph. Values represent the mean (± SD) of 4 independent experiments. Data were analyzed using the Student t test (∗, P < .05; ∗∗, P < .01). The down-regulation of PAG protein expression is shown.
PAG down-regulation enhanced both Src kinase activity and Ras activation. Primary human T cells (A) and Jurkat T cells (B) were transfected with siRNA oligonucleotides or the plasmid pCMS3-EGFP containing PAG shRNA, respectively. The down-regulation of PAG protein expression compared with the control is shown. Cells were stimulated with CD3 and CD28 for the times indicated, and lysates blotted with anti-phosphotyrosine. Actin staining is included to show equal loading. (C) In addition, the ability of transfected Jurkat cells to activate Ras was determined using the GST-Raf1-RBG pull-down assay. Total Ras is included as the control and the normalization of active/total Ras appears in the graph. Values represent the mean (± SD) of 4 independent experiments. Data were analyzed using the Student t test (∗, P < .05; ∗∗, P < .01). The down-regulation of PAG protein expression is shown.
Discussion
In our study of anergic T cells, we identified several novel interactions that connect increased Fyn kinase activity to the block in Ras activation. First, recall that Fyn phosphorylates PAG, recruiting Csk, which in turn phosphorylates the inhibitory tyrosine within Fyn. However, because of its association with PAG, Fyn cannot enter a closed-inactive conformation and thus remains active. At this point, two additional alterations are required. One involved a decrease in membrane phosphatase activity, either CD45 or RPTPα, toward the inhibitory tyrosine of Fyn to explain the specific increase in Y529 phosphorylation observed in anergic T cells (Figure 3A). This might involve degradation of the phosphatase, alteration of its enzymatic activity, or spatial segregation (eg, exclusion from the immune synapse). It is noteworthy that a similar phenotype (ie, increased Fyn kinase activity, PAG hyperphosphorylation, increased Csk recruitment, and inhibitory tyrosine phosphorylation) is seen in RPTPα-deficient mice.41 The other alteration involves the phosphorylation of Y215 within the SH2 domain of Fyn by an unknown kinase. It is through the increase in inhibitory tyrosine phosphorylation (Y529) together with phosphorylation of the SH2 domain (Y215) that Fyn enters into a hyperactive conformation, in that SH2 domain phosphorylation prevents binding of the inhibitory tyrosine.42 This is the first time that such a conformation has been described for Fyn or in human T cells. Increased Fyn kinase activity in turn led to the hyperphosphorylation of PAG (Figure 2) and potentially other Fyn substrates (Figure 1E), two of which are Sam68 and RasGAP.34,43
We are also the first to have shown that the expression of both Sam68 and RasGAP were increased in stimulated T cells, as well as to have identified the formation of a multiprotein complex containing PAG, Fyn, Sam68, and RasGAP (Figures 4 and 7). We believe that the increased Fyn kinase activity (Figure 2A) within anergic T cells may contribute to the stability of this complex, because several of the interactions are postulated to occur via SH2 domain binding. In these cells, Fyn binds to PAG via its SH3 domain12 and is therefore free to bind a phosphorylated Sam68 via its SH2 domain.44 Alternatively, RasGAP could perform this linker function in that it possessed 2 SH2 domains in addition to an SH3 domain and has been proposed to preferentially bind to PAG via its N-terminal SH2 domain and Sam68 by the C-terminal SH2 domain35,45 ; one function of Sam68 may be to stabilize RasGAP binding. The presence of this multimolecular complex (PAG, Fyn, Sam68, and RasGAP) within lipid rafts is ideally positioned to block Ras activation, because Ras-GDP is itself raft-localized.37 Therefore, an enhanced recruitment of RasGAP to this membrane compartment would stimulate the rapid hydrolysis of any Ras-GTP that forms, thus effectively shutting down Ras. Recently, the PAG-Fyn complex was shown to promote anergy in mice.46
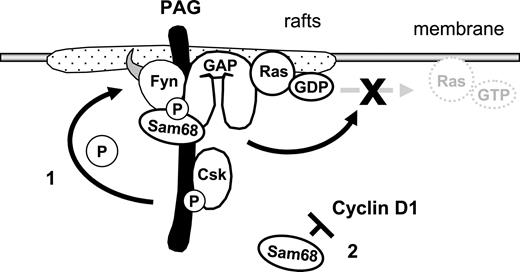
Model demonstrating how the PAG complex contributes to T-cell anergy. Enhanced PAG-phosphorylation by Fyn leads to increased recruitment and activity of Csk. This results in enhanced phosphorylation of Y529 within Fyn, which, together with the phosphorylation of Y215, further enhances Fyn kinase activity. This facilitates the phosphodependent recruitment of RasGAP onto PAG within the rafts, where it stimulates the intrinsic GTPase activity of Ras, thereby preventing the translocation of active Ras out of the rafts. Secondly, increased Sam68 binding may stabilize the complex and free mRNAs for anergy-inducing factors; also, increased Sam68 expression can block cyclin D1, thus preventing the G1/S transition.
Model demonstrating how the PAG complex contributes to T-cell anergy. Enhanced PAG-phosphorylation by Fyn leads to increased recruitment and activity of Csk. This results in enhanced phosphorylation of Y529 within Fyn, which, together with the phosphorylation of Y215, further enhances Fyn kinase activity. This facilitates the phosphodependent recruitment of RasGAP onto PAG within the rafts, where it stimulates the intrinsic GTPase activity of Ras, thereby preventing the translocation of active Ras out of the rafts. Secondly, increased Sam68 binding may stabilize the complex and free mRNAs for anergy-inducing factors; also, increased Sam68 expression can block cyclin D1, thus preventing the G1/S transition.
Indeed, our reconstitution experiments (Figure 5) showed that PAG is able to suppress Ras activation. Recall that PAG is an adaptor protein and therefore possesses no enzymatic activity, but functions as a scaffold recruiting other proteins to the membrane. However, the ability to suppress Ras is unique to PAG, in that the overexpression of LAT, an adaptor of similar structure, does not have this effect. While several groups have demonstrated that PAG is able to bind the SH2 domain of RasGAP in vitro,12,35 we are the first to have shown that this interaction does indeed occur in vivo (at Y181) and to have demonstrated that PAG can suppress Ras independently of its ability to recruit Csk. Thus, we are now able to propose that PAG not only negatively regulates the activity of Src kinases via recruiting Csk, but also Ras by recruiting GAPs to the membrane. Because both protein families are important for regulating cellular activation and oncogenesis, alterations in PAG expression or localization could be a contributing factor in cancer and autoimmunity.
In addition to its potential role as an adaptor within the PAG-Fyn complex, Sam68 belongs to the STAR (signal transduction and activation of RNA) family of RNA binding proteins.47,48 Its phosphorylation by Fyn is dependent on the binding of the Fyn SH3 domain to Sam68.49 Both SH3-domain binding and tyrosine phosphorylation correlate negatively with RNA binding and positively with localization to the cytosol.50,51 Because Sam68 influences the cell cycle,47 and anergic T cells are arrested at the G1/S transition,52 the increase in Sam68 expression may contribute to this block. Indeed, Sam68 overexpression results in cell cycle arrest in the G1 phase.53 Given that recent studies have questioned p27kip1 and p21cip as candidates for the block in cell cycle progression,54 it will be interesting to determine whether Sam68-deficient mice possess a defect in anergy induction or whether it is rather the combined action of multiple redundant mechanisms that keeps self-reactive T cells silent.
In summary, we present a simplified model of our findings (Figure 7). The multiprotein complex could contribute to the anergic phenotype in several ways. First, via the hyperphosphorylation of PAG and enhanced Csk recruitment, we observed increased phosphorylation of the inhibitory tyrosine within Fyn, which together with Y215 phosphorylation enhanced Fyn kinase activity. Second, via recruitment of RasGAP, this complex may contribute to the block in Ras activation that has become the hallmark of anergic T cells. Indeed, Ras activation is initiated via LAT and therefore the recruitment of GAPs into lipid rafts would be an effective means of inhibiting Ras. Finally, the increase in Sam68 expression could block cyclin D1, which is required for the transition into S phase. Together, we have identified two mechanisms by which T cells could be held in check. Certainly these are not the only mechanisms responsible for anergy, in that the contribution of E3 ubiquitin ligases is required.55 Therefore, it seems that T cells have developed multiple mechanisms to ensure that signaling events are tightly regulated. Here, we have shown that PAG is important for negatively regulating multiple components of TCR signaling and that by suppressing T-cell activation, it may contribute to anergy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Ignacio Rubio for help with the Ras activation assay, Bernhard Reis and Dr Sandra Beer for help with subcellular fractionation, and Drs Luca Simeoni and Sabine Lindquist for helpful discussion.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG)[JL 1031/1-1]. M.S. and A.P-F. were supported in part by a grant from the German Ministry for Education and Research (BMBF) NBL-3 program 01ZZ0407. V.H. is supported by Center of Molecular and Cellular Immunology grants 1M0506 and AV0Z50520514.
Authorship
Contribution: M.S. performed experiments and made the figures, A.P-F. and V.H. contributed vital reagents, B.S. and J.L. designed research and analyzed results, and M.S. and J.L. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interest.
Correspondence: Jonathan A. Lindquist, Institute of Immunology, Otto-von-Guericke University, Leipziger Str. 44, 39120 Magdeburg, Germany; e-mail: Jon.Lindquist@med.ovgu.de.

![Figure 1. Anergic T cells proliferate only in response to exogenous IL-2 and show markedly enhanced Fyn kinase activity. (A) Peripheral T cells were pretreated with no stimulus (resting), anti-CD3 antibody (anergic), or anti-CD3 antibody plus phorbol ester PMA (rescued) for 3 days. After 1 day of resting, the cells received either no stimulus or were restimulated with anti-CD3, anti-CD3 + anti-CD28, or anti-CD3 + IL-2. Incorporation of [3H]thymidine was measured at 72 hours and is shown as the mean value of triplicates (± SD). Data were analyzed using one-way ANOVA (∗∗, P < .01; ∗∗∗, P < .001). Fyn (B) and Lck (C) were immunoprecipitated from resting (rest.), anergic (aner.), and rescued (resc.) T cells and in vitro kinase assays (IVK) were performed. Phosphorylation was visualized by autoradiography (upper panel), the amount of kinase precipitated was determined by immunoblotting with specific antibodies (lower panel). Phosphorylation of the exogenous substrate enolase and Fyn autophosphorylation were normalized with respect to the level of kinase. These values are presented here as the fold-induction (FI). (D) Expression of Fyn and Lck from total lysate. Actin shows equal loading. (E) Resting, anergic and rescued cells were either left untreated (−) or stimulated via CD3 (MEM-92) for 2 minutes (+), lysed, and subjected to Western blotting. Changes in protein tyrosine phosphorylation are shown. Equal loading is shown with actin staining. (F) To better visualize the defects in proximal signaling, the TCR ζ chain and LAT were immunoprecipitated. Western blots of anti-phosphotyrosine staining and total protein are presented.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-07-038752/4/m_zh80130703890001.jpeg?Expires=1763733920&Signature=i9fRJFcF-xQ2-MozT6AeUxyyC2BTAASDLdIR4fRzeXE25ZsoROOSfP6TzA2ni9lmeb2gfHSQddUZjFxXBw9S5Nt70ltLGyAMMN9Oa1V-bBTb4xRCxdwEbTbh7lcP2KDNlV2d0lPqzSEXR1nyso23e3dWbHqL6DGqjgjPPjaSIIclJRUYq2tPcFnREMkp-AqJfxOqFytEbZhRJB-2hnqauMi8b0pwFLHpXAPCr-mnvnxRVn2xy45eqRqloWirkBc7Fb74FZG9rUf76KTYTXWXrpll~txpyyqeKG8Hk9cKi~kJntPeYguXeoP6UXnlrSzNQF2CHLsvz579i3dTh1ePmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal