A prospective multicenter trial was conducted to evaluate the safety and feasibility of granulocyte colony-stimulating factor (G-CSF)–primed bone marrow (G-BM) in children receiving allogeneic bone marrow transplantation (BMT). A total of 42 children with a median age of 9.8 years (range, 0.8-17 years) were enrolled. Donors with median age of 9.2 years (range, 1.1-22 years) received 5 μg/kg per day of subcutaneous G-CSF for 5 consecutive days. BM was harvested on the fifth day. No donor experienced complications related to G-CSF administration or marrow har-vest. Median nucleated (NC) and CD34 cells infused was 6.7 × 108/kg (range, 2.4-18.5 × 108/kg) and 7.4 × 106/kg (range, 2-27.6 × 106/kg), respectively. Neutrophil and platelet engraftment was at a median of 19 days (range, 13-28 days) and 20 days (range, 9-44 days), respectively. A total of 13 (32%) patients developed grade 2 graft-versus-host disease (GVHD), and 5 (13%) of 40 evaluable patients developed chronic GVHD (3 limited and 2 extensive). Higher cell dose was not associated with increased risk of acute or chronic GVHD. Overall survival and event-free survival at 2 years were 81% and 69%, respectively. Collection of G-BM from pediatric donors is safe, and can result in high NC and CD34 cell doses that facilitate engraftment after myeloablative BMT without a discernable increase in the risk of GVHD.
Introduction
Bone marrow transplantation (BMT) is an important therapeutic intervention in children and adults with malignant and nonmalignant disorders. Although for many years the most commonly used hematopoietic stem-cell source has been BM, in recent years peripheral blood stem cells (PBSCs) mobilized with granulocyte colony-stimulating growth factor (G-CSF) have become an alternative source of hematopoietic stem cells. In initial clinical trials, PBSCs have been shown to offer the advantage of higher stem cell dose and faster engraftment than unmodified BM.1 PBSCs have become the preferred stem-cell source for most adults receiving HLA-identical sibling donor transplantation, but are not as widely accepted as a standard stem-cell source in pediatric patients. In a recent analysis by the International Bone Marrow Transplant Registry (IBMTR), the use of PBSCs in pediatric patients was associated with higher risk of chronic graft-versus-host disease (GVHD) and transplantation-related mortality.2 In addition, apheresis of young children is often challenging, and may result in blood product exposure and central line placement for the donor.3
Several studies have shown that higher BM cell dose is associated with more rapid engraftment, less risk of fatal GVHD, and improved survival in both related and unrelated marrow transplantations.4,5 Treatment of the BM donor with G-CSF prior to the harvest results in a significant increase of total nucleated cell (TNC) count and CD34+ progenitor cells. Studies in adults using G-CSF–primed BM (G-BM) harvest have shown improved engraftment kinetics compared with conventional marrow, similar to PBSCs, without increased incidence of chronic GVHD.6,–8 Thus, G-BM may offer some or all of the benefits of PBSCs compared with conventional BM, with fewer of the risks of PBSCs. We conducted a prospective multicenter pilot study to evaluate the safety and feasibility of G-BM in pediatric patients receiving HLA-identical sibling donor marrow transplants.
Patients and methods
Patient accrual and characteristics
Between February 2003 and November 2005, pediatric patients undergoing allogeneic transplantation from an HLA-identical sibling were asked to participate in a pilot study of G-BM. The study was approved by the Pediatric Blood and Marrow Transplant Consortium (PBMTC) scientific committee and the institutional review board of each participating institution, and monitored by the PBMTC Data Safety Monitoring Committee. An informed consent was obtained from all patients, donors, or their legal guardians in accordance with the Declaration of Helsinki.
BM collections
All donors received 5 μg/kg per day of G-CSF (filgrastim; Amgen, Thousand Oaks, CA) as a single subcutaneous injection for 5 consecutive days. G-BM harvest was performed on the fifth day with a target volume of 15 to 20 mL/kg of patient's weight, not to exceed 20 mL/kg of donor's weight. The BM was infused the same day it was collected. Plasma or red cell depletion was performed if there was any ABO incompatibility, following institutional guidelines.
Evaluations and definitions
Bone marrow product and donor peripheral blood were analyzed for CD34+ subsets and T-cell subsets by flow cytometry using previously published methods.9 Growth factor use was not permitted unless the absolute neutrophil count (ANC) was less than 0.5 × 109/L on day 21 after transplantation. Neutrophil engraftment was defined as the first of 3 days with an ANC greater than 500 cells/mm3 after the posttransplantation nadir. Platelet engraftment was defined as the first of 7 consecutive days with a platelet count greater than 20 × 109/L without platelet transfusions. Patients receiving a transplant for hematologic malignancies beyond first complete remission were considered “high risk,” and all others were considered “standard risk.” Acute and chronic GVHD were graded by Seattle criteria.10,11 Patients who died while in relapse after transplantation were categorized as having died of relapse. Patients who died without disease recurrence were categorized as experiencing nonrelapse mortality.
Donors were evaluated during the days of G-CSF administration, following BM collection, and 30 days later for any adverse events attributable to G-CSF.
Statistics
The primary aim of this study was to evaluate safety and feasibility of G-CSF administration to pediatric donors. Additional study outcomes include overall and event-free survival; time to neutrophil and platelet engraftment; incidence of acute and chronic GVHD; disease risk classification (high vs standard risk); and TNC, CD34, and CD3 cell counts. In addition, each patient was classified as standard risk or high risk according to the definition given in “Evaluations and definitions.”
The associations between risk classification and overall and event-free survival were evaluated by Kaplan-Meier estimates of survival and log-rank tests.12 Associations between transplantation factors and survival were evaluated using the Cox proportional hazards regression, and associations with continuous outcomes (eg, time to engraftment and CD34) were evaluated using the nonparametric Spearman correlation coefficient.13,14 All tests were judged significant at the .05 level.
Results
Study population
Characteristics of the 42 patients are shown in Table 1. There was a predominance of patients with hematologic malignancies and 17 patients had high-risk disease. Of those patients, 5 had received 1 (N = 4) or 2 (N = 1) prior transplantations. Median follow-up time of surviving patients was 886 days (range, 510-1391 days). Median donor age was 9.2 years (range, 1.1-22 years). All donors received the prescribed doses of G-CSF without any adverse events except for grade 1 skeletal pain reported in 2 donors that resolved with acetaminophen. None of the donors required hospitalization related to the G-CSF administration.
Patient and transplantation characteristics
| Characteristic . | Quantity . |
|---|---|
| Patients, no. | 42 |
| Median age, y (range) | 9.8 (0.8-17) |
| Sex, male/female, no. | 22/20 |
| Diagnosis, no. patients | |
| High-risk | |
| ALL, CR 2* or more | 5 |
| AML, CR 2/refractory* or more | 6 |
| Advanced MDS/JMML | 4 |
| NHL CR 2 | 2 |
| Standard-risk | |
| AML CR 1 | 9 |
| ALL CR 1 | 2 |
| CML CP 1 | 2 |
| MDS-RA | 3 |
| Severe aplastic anemia | 6 |
| Sickle cell disease | 1 |
| Red cell aplasia | 1 |
| Metabolic disorder | 1 |
| Preparative regimen, no. patients | |
| TBI/Cy with or without VP-16 | 12 |
| BU/CY with or without other | 24 |
| CY/ATG | 6 |
| GVHD prophylaxis†, no. patients | |
| CSP/MTX | 26 |
| FK/MTX | 7 |
| CSP or MTX | 4 |
| CSP/prednisone | 1 |
| FK/MMF | 3 |
| Characteristic . | Quantity . |
|---|---|
| Patients, no. | 42 |
| Median age, y (range) | 9.8 (0.8-17) |
| Sex, male/female, no. | 22/20 |
| Diagnosis, no. patients | |
| High-risk | |
| ALL, CR 2* or more | 5 |
| AML, CR 2/refractory* or more | 6 |
| Advanced MDS/JMML | 4 |
| NHL CR 2 | 2 |
| Standard-risk | |
| AML CR 1 | 9 |
| ALL CR 1 | 2 |
| CML CP 1 | 2 |
| MDS-RA | 3 |
| Severe aplastic anemia | 6 |
| Sickle cell disease | 1 |
| Red cell aplasia | 1 |
| Metabolic disorder | 1 |
| Preparative regimen, no. patients | |
| TBI/Cy with or without VP-16 | 12 |
| BU/CY with or without other | 24 |
| CY/ATG | 6 |
| GVHD prophylaxis†, no. patients | |
| CSP/MTX | 26 |
| FK/MTX | 7 |
| CSP or MTX | 4 |
| CSP/prednisone | 1 |
| FK/MMF | 3 |
ALL indicates acute lymphocytic leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; JMML, juvenile myelomonocytic leukemia; NHL, non-Hodgkin lymphoma; CR, completre response; CP, chronic phase; MDS-RA, MDS-refractory anemia; VP-16, etoposide; BU, busulfan; CY, cyclophosphamide; TBI, total body irradiation (1200–1320 cGy); CSP, cyclosporine; FK, tacrolimus; MTX, methotraxate, and ATG, antithymocyte globulin.
Of these patients, 5 underwent prior allogeneic transplantations.
One patient received a syngeneic transplant and had no GVHD prophylaxis.
BM product
The median TNC and CD34+ cell counts were 6.7 × 108/kg (range, 2.4-18.6 × 108/kg) and 7.4 × 106/kg (range, 2.0-27.6 × 106/kg) of recipient weight, respectively. The median number of CD3+ cells infused was analyzed in 34 patients and was 26.9 × 106/kg (range, 1.5-74 × 106/kg). To assess for potential contamination of the collected bone marrow by peripheral blood, the absolute number of CD34+ cells was measured in the blood and BM on the day of the collection in 30 donors. The absolute CD34+ cell count was significantly higher in BM compared with peripheral blood, with a median of 513 cells/μL (range, 116-1005 cells/μL) versus 50 cells/μL (range, 8-247 cells/μL), respectively (P < .001).
Engraftment
All patients achieved neutrophil engraftment at a median time of 19 days (range, 13-28 days). Median time to platelet recovery was 20 days (range, 9-44 days). A total of 2 patients were excluded from the analysis; 1 patient relapsed early and went to receive salvage therapy, and another died of complications prior to reaching platelet recovery. A patient with severe aplastic anemia experienced secondary graft failure 1 year after transplantation, received a second transplant from the same donor, and is alive with complete donor engraftment. There is significant association between TNC count and both neutrophil and platelet engraftment. Doubling of the TNC count was associated with a 2.5-day reduction in time to neutrophil engraftment (P = .01) and a 5-day reduction in time to platelet engraftment (P = .005). Neither platelet nor neutrophil engraftment was associated with CD34+ cell count.
Acute and chronic GVHD
A total of 13 of 41 patients developed grade 2 acute GVHD with a cumulative incidence of 32% (95% confidence interval [CI]: 18-46). Grades 3 to 4 acute GVHD was not observed. While higher TNC count was not associated with acute GVHD (P = .26), a lower CD34+ cell count was associated with increased risk of grade 2 acute GVHD (P = .02). Median CD34 count for patients with or without acute GVHD was 6.0 × 106/kg versus 10.5 × 106/kg. There was no association between CD3 count and acute GVHD (P = .13).
A total of 5 of 40 evaluable patients developed chronic GVHD with a cumulative incidence of 13% (95% CI: 2-23). Of these, 2 patients developed extensive and 3 developed limited chronic GVHD. There was no association between higher TNC count, CD34+ cell count, and chronic GVHD (P = .15 and P = .6, respectively). There was no association between CD3+ cell count and chronic GVHD (P = .96).
Relapse and survival
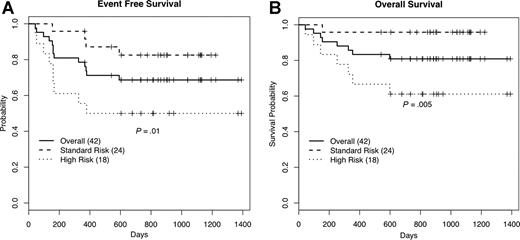
A total of 11 patients relapsed, 7 with high-risk disease and 4 with standard-risk disease. Of these, 8 patients died secondary to disease relapse and 3 patients are alive without disease after salvage chemotherapy. A total of 2 patients died of transplantation-related complications; 1 from sepsis in the setting of acute GVHD, and another from multisystem organ failure. The estimated event-free survival (EFS) and overall survival at 2 years was 69% (95% CI: 56-84) and 81% (95% CI: 70-94), respectively. The EFS was significantly better among standard-risk patients compared with high-risk patients, 83% (95% CI: 69-100) versus 50% (95% CI: 32-79) (P = .01). Similarly, overall survival was significantly better among standard-risk compared with high-risk patients, 96% (95% CI: 88-100) versus 61% (95% CI: 42-88) (P = .005; Figure 1).
Kaplan-Meier survival estimates. Percentages of event-free (A) and overall (B) survival at 24 months.
Kaplan-Meier survival estimates. Percentages of event-free (A) and overall (B) survival at 24 months.
Discussion
A number of groups have recently reported on the outcome of patients who received G-CSF–mobilized PBSCs during HLA-matched allogeneic transplantation.1,15,–17 These results have consistently shown that use of PBSCs as a stem-cell source led to more rapid engraftment of both neutrophils and platelets compared with BM. In a randomized trial, the Seattle group reported that allogeneic PBSCs resulted in significantly faster neutrophil and platelet engraftment and improved survival without increase in the incidence of acute or chronic GVHD with a relatively short follow-up of 1 year.1 This trial included only few pediatric patients, since only donors who weighed more than 40 kg were included. Other groups have reported significantly higher risk of chronic GVHD in recipients of PBSC transplants.15,–17 Despite the lack of prospective randomized trials of PBSCs in children, a recent study from the PBMTC group revealed that 23% of all allogeneic-matched sibling transplantations in children used PBSCs as the source for the transplant.18 Results from 3 separate studies in children using PBSCs as a stem-cell source in matched-related donor transplantations have shown a chronic GVHD disease rate of 63% to 75%,19,–21 twice that of what is expected in pediatric patients receiving unstimulated BM.2,22
Recently, a retrospective analysis was performed by the IBMTR of 143 PBSC and 630 BM transplantations from HLA-identical sibling donors in children aged 8 to 20 years with acute leukemia. This analysis revealed significantly higher rates of chronic GVHD (P = .001) and transplantation-related mortality (P = .001) in recipients of PBSCs, with recipients of BM having significantly better survival than PBSC recipients (P = .01).2
There have been several small single-arm and randomized studies of G-BM in adult recipients. Isola et al reported on 10 patients who received G-CSF–stimulated allogeneic BM and compared their results with historic BM recipients. The G-BM group attained neutrophil engraftment 9 days earlier and platelet engraftment 6 days earlier than historic controls receiving unstimulated HLA-identical sibling BM.7 Couban reported on 29 allogeneic transplantations using G-BM and showed that platelet and neutrophil engraftment was faster than historic controls with unstimulated marrow, but both groups had similar lengths of hospital stays, febrile days, and days on antibiotics.6 A prospective randomized trial comparing G-BM versus G-PBSCs found no significant difference in neutrophil or platelet engraftment, but a significantly increased risk of acute (17% vs 46%) and chronic (27% vs 77%) GVHD in recipients of PBSCs.8 Similar results were reported by Serody et al.23
We report the first multicenter experience using G-CSF–primed BM from pediatric donors as a source of stem cells for transplantation in children. Our study demonstrates the safety of the use of G-CSF in pediatric donors. The youngest donor in our study was 1 year old, and only 1 donor was older than 18 years of age. Donors tolerated G-CSF administration without reported symptoms except for mild, reversible skeletal pain. Previous studies of G-CSF in pediatric donors have reported a rate of skeletal pain ranging from 11% to 17.5%.3,21 This higher incidence of pain is probably related to the higher dose of G-CSF (10 μg/kg) used for PBSC mobilization in these trials. In a recent study by the PBMTC, 97% of donors younger than 6 years and 67% of donors 7 to 12 years of age required central line placement for PBSC collection.3 The risk of central line placement in otherwise healthy pediatric donors should be balanced against the risk of BM harvest, since both have the risk of general anesthesia.
This trial demonstrates that priming with a lower dose of G-CSF (5 μg/kg) results in nucleated and CD34+ cell yields that are comparable with PBSC collections and greater than that achieved in BM collections, while avoiding the high CD3+ cell collections typical for PBSCs.1,16 The number of CD3+ cells collected was similar to unstimulated BM and 10-fold lower than that observed with PBSCs.1,8 In addition, the high number of nucleated and CD34+ cell count concentrations observed might allow the collection and successful transplantation of smaller BM volume, thus decreasing the morbidity in small pediatric donors. This approach might also be helpful in increasing the number of cells infused when pediatric donors are significantly younger or smaller than the recipients. Time to neutrophil engraftment in our study was longer than that previously reported with G-BM, but our patients did not receive posttransplantation growth factor support, while other studies used posttransplantation growth factor. Platelet engraftment for our patients was faster than that reported in other pediatric trials.2
Our study further demonstrates that infusing higher number of nucleated cells and CD34+ cells is not associated with an increased risk of acute or chronic GVHD. Of note, none of the patients in our study developed grade 3 or 4 acute GVHD. Compared with other pediatric studies, the incidence of chronic GVHD in our study is much lower than that observed using PBSCs in children.2,19,–21
The use of a higher nucleated cell dose in the BM has been associated with improved survival.4 G-BM can provide a potentially safer alternative for higher cell dose without increasing the risk of GVHD and transplantation-related mortality observed in children receiving PBSCs.
Our study has provided sufficient data to conclude that G-BM is a safe and feasible source of stem cells from related pediatric donors. These promising results have led to the development of ASCT0631, a Children's Oncology Group phase 3 randomized trial comparing G-BM with unstimulated marrow, which opened in late 2007.
Presented in abstract form at the 48th annual meeting of the American Society of Hematology, Orlando, FL, December 11, 2005.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
All participating institutions in the Pediatric Blood and Marrow Transplant Consortium (PBMTC) study are listed in the affiliations note on the title page of this article.
Authorship
Contribution: H.F., J.E.L., E.R.N., A.W., and S.A.G. designed the study. H.F. and D.B. performed data analysis. All authors participated in study conduct, interpretation of data, and approval of final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Haydar Frangoul, Vanderbilt University, 2200 Pierce Ave, 397 PRB, Nashville, TN 37232-2573; e-mail:haydar.frangoul@vanderbilt.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal