Leukemia is thought to arise from malignant stem cells, which have been described for acute and chronic myeloid leukemia (AML and CML) and for acute lymphoblastic leukemia (ALL). Leukemia stem cells (LSCs) are relatively resistant to current chemotherapy and likely contribute to disease relapse and progression. Consequently, the identification of drugs that can efficiently eradicate LSCs is an important priority. In the present study, we investigated the antileukemia activity of the compound TDZD-8. Analysis of primary AML, blast crisis CML (bcCML), ALL, and chronic lymphoblastic leukemia (CLL) specimens showed rapid induction of cell death upon treatment with TDZD-8. In addition, for myeloid leukemias, cytotoxicity was observed for phenotypically primitive cells, in vitro colony-forming progenitors, and LSCs as defined by xenotransplantation assays. In contrast, no significant toxicity was observed for normal hematopoietic stem and progenitor cells. Notably, cell death was frequently evident within 2 hours or less of TDZD-8 exposure. Cellular and molecular studies indicate that the mechanism by which TDZD-8 induces cell death involves rapid loss of membrane integrity, depletion of free thiols, and inhibition of both the PKC and FLT3 signaling pathways. We conclude that TDZD-8 uses a unique and previously unknown mechanism to rapidly target leukemia cells, including malignant stem and progenitor populations.
Introduction
In the hematopoietic system, stem cells (HSCs) are essential for homeostasis, whereby HSCs self-renew, proliferate, and differentiate into all of the mature blood cell types. Recently, it was shown that myeloid and some forms of lymphoid leukemia are induced by the malignant transformation of primitive hematopoietic cells, giving rise to leukemic stem cells (LSCs).1,,–4,8 LSCs have similar characteristics as HSCs in terms of self-renewal and proliferation; however, their developmental program is aberrant, giving rise predominantly to undifferentiated leukemic blasts. LSCs are found in a quiescent state and may overexpress multidrug efflux pumps5,6,9,10 ; features that render them resistant to conventional chemotherapy agents.7,11,,–14 Because LSCs have the capacity to regenerate malignant blast cells, failure to effectively ablate this population may lead to disease progression, relapse after therapy, or both.15,16 Moreover, standard chemotherapy is inhibitory to normal stem and progenitor cells, often resulting in serious myelosuppression and impairment of hematopoietic functions.17,18 Therefore, given the central role of LSCs in leukemia pathogenesis and the importance of normal HSCs in generation of mature blood cells, we have focused on the identification of compounds that have the capacity to eradicate LSCs without harming normal hematopoietic stem and progenitor populations.
In our previous work, we have exploited unique molecular features of acute myeloid leukemia stem cells (AML-SCs) as a means to develop strategies for targeted therapy. One of these features is the constitutive activation of NF-κB, which is present in AML-SCs but not in normal HSCs.19 NF-κB plays a key role in inflammation and stress responses and is a major regulator of cell survival.20,21 Given the known role of NF-κB in the regulation of cancer cell survival, it represents a potentially important target for specific elimination of primitive leukemia cells. Successful strategies that use known NF-κB inhibitors include (1) the combination of proteasome inhibitor MG132 alone or in combination with the anthracycline idarubicin and (2) the sesquiterpene lactone parthenolide (PTL).14,19,22 Both approaches show selective targeting of AML stem and progenitor cells and are being pursued for clinical application.
In an effort to expand strategies for targeting primitive leukemia cells, we have recently explored inhibitors of various pathways implicated in early hematopoiesis. These studies included analysis of the compound TDZD-8 (4-benzyl,2-methyl,1,2,4-thiadiazolidine, 3,5 dione), which was originally developed as a non-ATP competitive inhibitor of GSK-3β.23,24 To date, TDZD-8 has been evaluated primarily as a cytoprotective agent in multiple rodent models for maladies such as septic and nonseptic shock, lung injury, arthritis, spinal cord injury, colitis, and Alzheimer disease.25,,,,,,,–33 However, in the present study we describe an entirely new activity for TDZD-8, which thus far appears to be restricted to cells derived from hematologic malignancies. We show that this compound is strongly cytotoxic to multiple types of primary leukemia cells, as well as phenotypically and functionally defined LSCs. This cytotoxicity is associated with a rapid loss of membrane integrity, induction of oxidative stress, and inhibition of several signal transduction pathways.
Materials and methods
Cell isolation and culture
Primary human AML, blast crisis chronic myeloid leukemia (bcCML), acute lymphoblastic leukemia (ALL), chronic lymphoblastic leukemia (CLL) cells, mobilized peripheral blood (MPB) and normal bone marrow (BM) cells were obtained from volunteer donors. Informed consent was obtained in accordance with the Declaration of Helsinki. All manipulation and analysis of specimens was approved by the University of Rochester Institutional Review Board. Additional samples were obtained from the Quebec Leukemia Cell Bank, which collects specimens from 10 university and regional hospitals. Umbilical cord blood (CB) was obtained from the National Disease Research Interchange, or with informed consent from volunteer donors at Rochester General Hospital. Mononuclear cells were isolated from the samples using Ficoll-Paque (Pharmacia Biotech, Piscataway, NY) density gradient separation. In some cases cells were cryopreserved in freezing medium of Iscove modified Dulbecco medium, 40% fetal bovine serum (FBS), and 10% dimethylsulfoxide or in CryoStor CS-10 (VWR). Cells were cultured in serum-free medium (SFM)34 for 1 hour before the addition of drugs. TDZD-8 and parthenolide were obtained from EMD Chemicals (San Diego, CA) and Biomol (Plymouth Meeting, PA), respectively.
Flow cytometry
Apoptosis assays were performed as described.19 Briefly, after 18 to 24 hours of treatment, specimens were labeled using anti–CD38-allophycocyanin (APC), CD34-PECy7, CD123-phycoerythrin (PE), or CD10–fluorescein isothiocyanate (FITC) (Becton Dickinson, San Jose, CA) for 15 minutes. Cells were washed in cold PBS and resuspended in 200 μL of annexin-V buffer (0.01 M HEPES/NaOH, 0.14 M NaCl, 2.5 mM CaCl2), Annexin V–FITC, or Annexin V–PE (Becton Dickinson) and 7-aminoactinomycin (7-AAD; Molecular Probes, Eugene, OR). Samples were then incubated at room temperature for 15 minutes and analyzed on a BD LSRII flow cytometer. Analyses for phenotypically described stem cell subpopulations were performed by gating CD34+CD38−CD123+, CD34+CD10−, and CD34+CD38− for AML, ALL, and normal specimens, respectively. To assess human cell engraftment in the nonobese diabetic/severe combined immunodeficient (NOD/SCID) xenotransplantation model, BM cells were blocked with the anti-Fc receptor antibody 2.4 G2 and 25% human serum and then labeled with anti–human CD45-PE antibody (Becton Dickinson). For leukemia specimens, CD34 and CD123 expressions were also evaluated. Free-thiol analysis was performed by labeling cells with monobromobimane (mBBr; Probes-Invitrogen, Carlsbad, CA). Phospho-FLT3 (Tyr591) Alexa Fluor-488 conjugate (Cell Signaling, Danvers, MA) was used for detection of active FLT3. For multispectral imaging flow cytometry, cells were stained with YoPro-1 (Probes-Invitrogen), 7-AAD, Draq 5, and CD45-PE. Membrane integrity assays were also performed by standard flow cytometry by staining with YoPro-1, Hoescht-33342, propidium iodide (PI) (Probes-Invitrogen), and annexin V–APC. Cells were analyzed using the Amnis Imagestream imaging cytometer (Amnis, Seattle, WA).
Methylcellulose colony-forming assay
Specimens were cultured in SFM as above at different time points in the presence or absence of TDZD-8. Cells were then plated at 50 000 cells/mL in Methocult GFH4534 (Stem Cell Technologies, Vancouver, BC) supplemented with erythropoietin 3 U/mL and G-CSF 50 ng/mL. Colonies were scored after 10 to 14 days of culture.
NOD/SCID mouse assays
NOD/SCID mice were sublethally irradiated with 2.7 Gy (270 rad) using a RadSource-2000 x-ray irradiator before transplantation. Leukemia cells for NOD/SCID analysis were obtained from PB specimens, and normal cells were obtained from CB specimens. Cells to be assayed were injected into the tail vein (5-10 million cells) in a final volume of 0.2 mL of PBS with 0.5% FBS. After 6 to 8 weeks, animals were killed, and BM was analyzed for the presence of human cells by flow cytometry.
Kinase assays
A commercial kinase profiling service was used for single point and titration assays.
Immunoblots
Cells were prepared and analyzed as previously described.35 Membrane fractions were prepared using mem-PER eukaryotic membrane protein extraction kit as per the manufacturer's instructions (Pierce, Rockford, IL). Blots were probed with phospho-PKC (pan) (βII; Ser660), phospho-PKCα/βII (Thr638/641), total PKCα, PKCβ, and FLT3 (Santa Cruz Biotechnology, Santa Cruz, CA), caspase-3 (Cell Signaling Technologies, Danvers, MA), caspase-8 and PARP (BD Bioscience), cleaved PARP (abcam), or antiactin (AC-15; Sigma, St Louis, MO) antibodies.
Statistical analysis
Statistical analyses and graphs were performed using GraphPad Prism software (GraphPad Software, San Diego, CA). Data were log transformed and analyzed by 1-way ANOVA followed by Tukey post hoc test. For 2 group comparisons, significance was determined by paired t tests.
Results
TDZD-8 induces leukemia-specific cell death
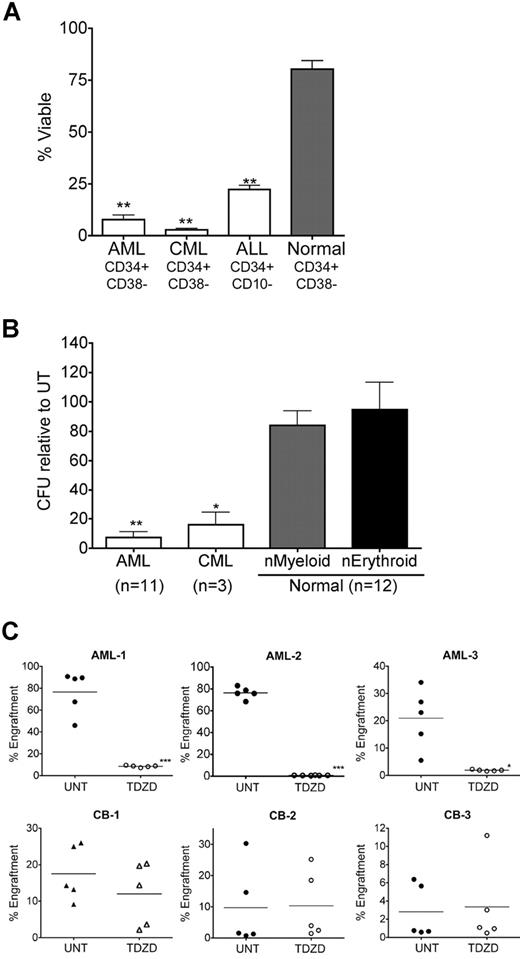
Initial studies were performed to determine the effects of TDZD-8 on different types of primary human leukemia (AML, bcCML, CLL, and ALL), as well as normal hematopoietic cells. Figure 1 shows the percent viability relative to untreated controls for primary human specimens treated with 20 μM TDZD-8 for 24 hours. All forms of leukemia were strongly impaired by TDZD-8, with mean viability of 15% for AML (n = 37), 7.2% for CLL (n = 12), 12.4% for ALL (n = 6), and 21.6% for bcCML (n = 6). In contrast, the cell viability for normal specimens was 79.5% (n = 13). Moreover, the lack of toxicity toward normal specimens was not significantly different for CB, BM, and MPB when each tissue type was analyzed separately (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, the cytotoxicity of TDZD-8 was significantly (P < .001) more specific to leukemia specimens. Given the broad efficacy toward leukemia cells, we further determined the range of activity for different types of tumor cells by submitting TDZD-8 for screening against the NCI-60 panel.36 Interestingly, TDZD-8 activity was specific to cell lines derived from hematologic malignancies, in which the average concentration to achieve 50% growth inhibition (GI50) was 8.3 μM (Table 1). All other tumor lines showed no growth inhibition up to concentrations of 100 μM. Together, these data indicate that, although TDZD-8 is highly cytotoxic to leukemia and related diseases, the compound does not substantially harm normal hematopoietic cells or tumors derived from other nonhematopoietic tissues.
TDZD-8 specifically induces cell death of primary leukemia specimens. (A) Primary AML (n = 37), CLL (n = 12), ALL (n = 6), bcCML (n = 6), and normal mononuclear cells (n = 13, obtained from BM [n = 3], CB [n = 7], or MPB [n = 3]) were cultured for 18 to 24 hours in the presence of 20 μM TDZD-8. Cell viability was assessed by Annexin V/7-AAD staining. Percent viability is represented relative to untreated control. Leukemia specimens were significantly (P < .001) more sensitive to TDZD-8 than were normal specimens. Error bars represent the SEM. All assays were performed in triplicate. (B) Representative dot plots for the 24-hour flow cytometric analysis with Annexin V/7-AAD stain in the presence or the absence of 20 μM TDZD-8.
TDZD-8 specifically induces cell death of primary leukemia specimens. (A) Primary AML (n = 37), CLL (n = 12), ALL (n = 6), bcCML (n = 6), and normal mononuclear cells (n = 13, obtained from BM [n = 3], CB [n = 7], or MPB [n = 3]) were cultured for 18 to 24 hours in the presence of 20 μM TDZD-8. Cell viability was assessed by Annexin V/7-AAD staining. Percent viability is represented relative to untreated control. Leukemia specimens were significantly (P < .001) more sensitive to TDZD-8 than were normal specimens. Error bars represent the SEM. All assays were performed in triplicate. (B) Representative dot plots for the 24-hour flow cytometric analysis with Annexin V/7-AAD stain in the presence or the absence of 20 μM TDZD-8.
NCI-60 screen
| Cell lines . | Log10 GI50 . | μM . |
|---|---|---|
| Leukemia | ||
| CCRF-CEM | −4.94 | 11.5 |
| HL-60 | -6.63 | 0.2 |
| K-562 | −5.19 | 6.5 |
| RPMI-8226 | -4.63 | 23.4 |
| SR | −8 | 0.01 |
| Non–small-cell lung cancer, 9 cell lines | -4 | >100.0 |
| Colon cancer, 7 cell lines | -4 | >100.0 |
| CNS cancer, 6 cell lines | -4 | >100.0 |
| Melanoma, 8 cell lines | -4 | >100.0 |
| Ovarian cancer, 5 cell lines | -4 | >100.0 |
| Renal cancer, 7 cell lines | -4 | >100.0 |
| Prostate cancer, 2 cell lines | -4 | >100.0 |
| Breast cancer, 7 cell lines | -4 | >100.0 |
| Cell lines . | Log10 GI50 . | μM . |
|---|---|---|
| Leukemia | ||
| CCRF-CEM | −4.94 | 11.5 |
| HL-60 | -6.63 | 0.2 |
| K-562 | −5.19 | 6.5 |
| RPMI-8226 | -4.63 | 23.4 |
| SR | −8 | 0.01 |
| Non–small-cell lung cancer, 9 cell lines | -4 | >100.0 |
| Colon cancer, 7 cell lines | -4 | >100.0 |
| CNS cancer, 6 cell lines | -4 | >100.0 |
| Melanoma, 8 cell lines | -4 | >100.0 |
| Ovarian cancer, 5 cell lines | -4 | >100.0 |
| Renal cancer, 7 cell lines | -4 | >100.0 |
| Prostate cancer, 2 cell lines | -4 | >100.0 |
| Breast cancer, 7 cell lines | -4 | >100.0 |
CNS indicates central nervous system.
TDZD-8 antileukemia effects are observed at the progenitor and stem cell levels
Although many agents show efficacy toward bulk tumor populations, eradication of more primitive stem and progenitor cells can represent a significant challenge. Given the established role of LSCs in several forms of leukemia,1,,–4,37 we examined the effect of TDZD-8 on phenotypically described stem cells from AML, bcCML, and ALL specimens. Treatment with 20 μM TDZD-8 for 24 hours resulted in a mean viability of 7.6% for CD34+CD38− from AML specimens (n = 10), 2.8% for CD34+CD38− from bcCML specimens (n = 3), and 22.3% for CD34+CD10− from ALL specimens (n = 3) (Figure 2A). In contrast, the viability of CD34+CD38− cells from healthy specimens (n = 7) was 80.2% after TDZD-8 treatment (Figure 2A, ▓). Thus, the specificity of the compound for phenotypically described LSCs was highly significant (P < .001). To determine whether TDZD-8 could also target functionally defined myeloid progenitor cells, we performed methylcellulose colony assays. Figure 2B shows that the ability of normal specimens to form colonies was not substantially affected by treatment with 20 μM TDZD-8 (84.14% myeloid colonies and 94.79% erythroid colonies; n = 12). In contrast, a significant decrease in colony formation was observed for both AML and bcCML, with only 7.3% CFU for AML (n = 11; P < .001) and 16.1% CFU for bcCML (n = 3; P < .01) after TDZD-8 treatment. Notably, for 6 of the 11 AML samples assayed, no colonies whatsoever were evident after treatment with TDZD-8. Further, we analyzed stem cell activity for AML and normal specimens using the NOD/SCID xenotransplantation model.38 Analysis of 3 independent specimens showed that treatment of leukemic cells with 20 μM TDZD-8 resulted in a significant decrease in stem cell activity, with mean engraftment reduced from 76% to 8% (P < .001), 76% to 0.7% (P = .001), and 21% to 2% (P < .01), respectively (Figure 2C). In contrast, little to no effect on the engraftment of normal specimens (n = 3) was observed after treatment with TDZD-8. Together these data show that TDZD-8 significantly impairs leukemic but not normal hematopoietic progenitor- and stem-cell function.
TDZD-8 ablates leukemia progenitor and stem cells. (A) Primary AML (n = 10), ALL (n = 3), bcCML (n = 3), and normal specimens (n = 7, obtained from BM, CB, or MPB specimens) were cultured for 18 to 24 hours in the presence or absence of 20 μM TDZD-8. Cell viability was assessed by flow cytometry in CD34+CD38− populations for AML and bcCML (CML) and CD34+CD10− cells for ALL using Annexin V/7-AAD stain. Percent viability is represented relative to untreated control. Specificity to leukemia specimens was significant (**P < .01). Error bars represent the SEM. (B) Primary cells from AML (n = 11), bcCML (CML; n = 3), and normal specimens (n = 12) were treated for 18 hours in suspension culture, followed by plating in methylcellulose. Error bars represent the SEM. Percentage of colony-forming units (CFUs) are normalized to untreated controls. All assays were performed in triplicate. Specificity to leukemia specimens was significant (**P < .01, *P < .05). (C) Percentage of marrow engraftment for NOD/SCID mice that received a transplant with AML (top panels) or normal CB (bottom panels) cells after 18 hours of culture with or without 20 μM TDZD-8. Each circle or triangle represents a single animal analyzed at 6 weeks after transplantation. Each plot represents an independent AML or CB specimen. Mean engraftment is indicated by the horizontal bars. **P < .01; ***P < .001.
TDZD-8 ablates leukemia progenitor and stem cells. (A) Primary AML (n = 10), ALL (n = 3), bcCML (n = 3), and normal specimens (n = 7, obtained from BM, CB, or MPB specimens) were cultured for 18 to 24 hours in the presence or absence of 20 μM TDZD-8. Cell viability was assessed by flow cytometry in CD34+CD38− populations for AML and bcCML (CML) and CD34+CD10− cells for ALL using Annexin V/7-AAD stain. Percent viability is represented relative to untreated control. Specificity to leukemia specimens was significant (**P < .01). Error bars represent the SEM. (B) Primary cells from AML (n = 11), bcCML (CML; n = 3), and normal specimens (n = 12) were treated for 18 hours in suspension culture, followed by plating in methylcellulose. Error bars represent the SEM. Percentage of colony-forming units (CFUs) are normalized to untreated controls. All assays were performed in triplicate. Specificity to leukemia specimens was significant (**P < .01, *P < .05). (C) Percentage of marrow engraftment for NOD/SCID mice that received a transplant with AML (top panels) or normal CB (bottom panels) cells after 18 hours of culture with or without 20 μM TDZD-8. Each circle or triangle represents a single animal analyzed at 6 weeks after transplantation. Each plot represents an independent AML or CB specimen. Mean engraftment is indicated by the horizontal bars. **P < .01; ***P < .001.
TDZD-8 activity involves oxidative stress
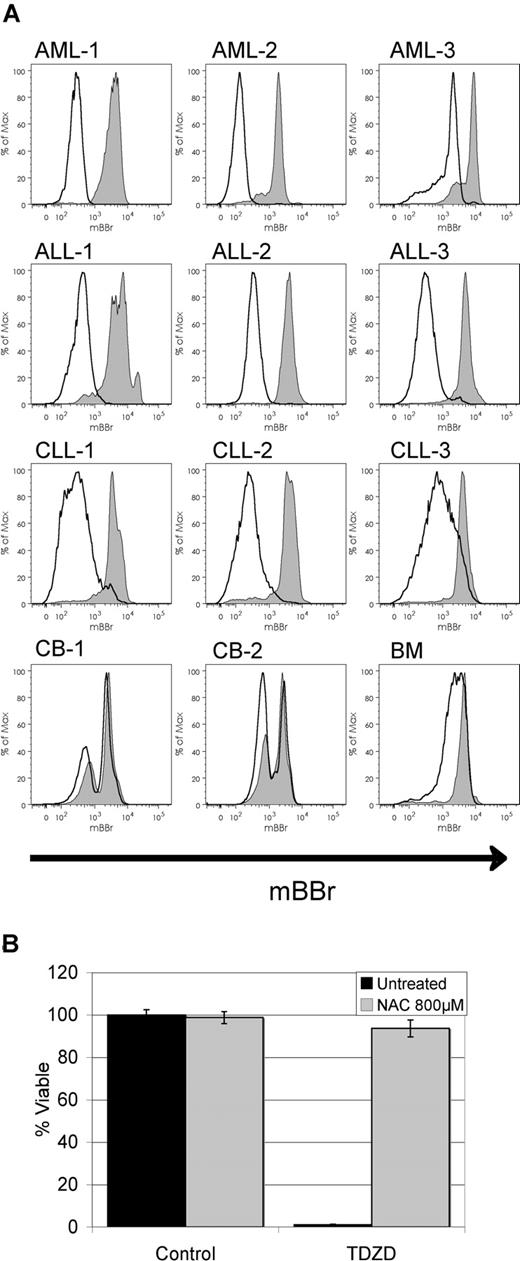
Previous studies have indicated that leukemia-specific agents may function by mechanisms involving the induction of oxidative stress.14 Therefore, we performed studies to examine whether TDZD-8 might also modulate the oxidative state of target cells. Shown in Figure 3A is labeling with the dye mBBr, which detects free thiol groups. Reduced labeling intensity signifies loss of free thiols, an indication of increased oxidative stress. Upon treatment with TDZD-8, reduction in mBBr labeling is evident in primary AML, ALL, and CLL specimens as early as 30 minutes after exposure, suggesting rapid thiol depletion in the cell. Interestingly, only slight changes in mBBr staining were observed in normal specimens, indicating that increased oxidative stress occurs in a leukemia-specific fashion. To further examine the role of oxidative state, we pretreated target cells with the antioxidant N-acetyl-cysteine (NAC), which completely blocked the cell death response in primary AML cells induced by TDZD-8 (Figure 3B). Taken together, these data indicate that TDZD-8 induces oxidative stress and that this activity is important for the antileukemia properties.
TDZD-8 treatment induces oxidative stress. (A) Flow cytometric overlays for mBBr fluorescence from primary AML, CLL, ALL, or normal mononuclear cells treated for 30 minutes with 20 μM TDZD-8 (black line) versus untreated controls (black line/gray filling). (B) Percentage of viability of primary AML cells pretreated with NAC (gray bars) for 1 hour before treatment with 20 μM TDZD-8. Viability was determined 24 hours after the addition of each drug. Error bars represent the SEM.
TDZD-8 treatment induces oxidative stress. (A) Flow cytometric overlays for mBBr fluorescence from primary AML, CLL, ALL, or normal mononuclear cells treated for 30 minutes with 20 μM TDZD-8 (black line) versus untreated controls (black line/gray filling). (B) Percentage of viability of primary AML cells pretreated with NAC (gray bars) for 1 hour before treatment with 20 μM TDZD-8. Viability was determined 24 hours after the addition of each drug. Error bars represent the SEM.
TDZD-8 antileukemia activity is observed with rapid kinetics
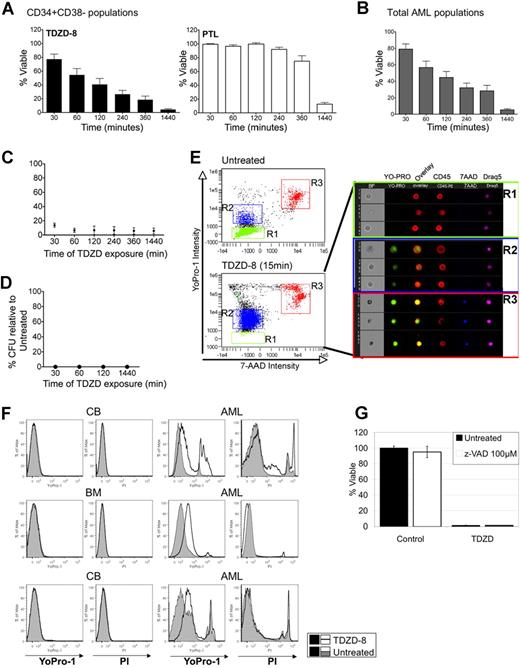
We noted that changes in oxidative state (Figure 3A) occurred with relatively rapid kinetics, suggesting that other cellular changes may also occur quickly. To further test the rate at which TDZD-8 may affect leukemic cells, we performed additional studies using primary AML specimens to determine viability at various times after exposure (0.5, 1, 2, 4, 6, and 24 hours). For comparison, parallel studies were performed with PTL, a drug we have previously shown can also specifically target primary human LSCs.14 As shown in Figure 4A (left panel), primitive AML CD34+CD38− cells treated with TDZD-8 displayed an extremely rapid loss of viability, with a mean time of only 2 hours to achieve 50% or more cell death. In contrast, PTL did not significantly change cell viability until 6 hours of treatment when the mean viability was still more than 70% (Figure 4A right panel). The rapid cell death induced by TDZD-8 treatment was also observed for bulk leukemia blast populations, in which an analysis of 17 primary specimens also showed a mean time of 2 hours to achieve 50% or more cell death (Figure 4B). Interestingly, although all 17 specimens responded relatively fast, 3 specimens showed a particularly dramatic reduction in viability to below 30% within 30 minutes. We also tested lymphoid specimens and observed rapid cell death kinetics for primary CLL and ALL samples (both total blast populations and phenotypically described stem cells) (Figure S2). Next, we examined the minimum time of exposure required for the commitment of AML populations to cell death. For these studies, cells were treated with 20 μM TDZD-8 for varying times and then immediately washed and replated in fresh culture medium. Cell viability was evaluated 24 hours after initial treatment. Strikingly, as little as 30 minutes of exposure to TDZD-8 was sufficient to commit primary human AML cells to death (Figure 4C). The 30-minute exposure time was also sufficient to inhibit the ability of AML progenitor cells to form colonies in methylcellulose culture (Figure 4D). Together, the data indicate that primary AML bulk and progenitor cell populations are irreversibly committed to cell death within 30 minutes of exposure to TDZD-8.
TDZD-8 induces cell death with extremely rapid cell death kinetics showing loss of membrane integrity. (A) Percent viability assessed at the indicated time points for CD34+CD38− populations of primary AML specimens (n = 8) treated with TDZD-8 (■) or PTL (□). Percent viability is represented relative to untreated control. (B) Percent viability assessed at the indicated time points for unfractionated primary AML specimens (n = 17) treated with TDZD-8. Percent viability is shown relative to untreated controls. Error bars represent SEM. (C) Cells were treated with 20 μM TDZD-8 for the indicated periods of time, then washed and placed in culture until analysis at 24 hours. Percent viability represented relative to untreated control. (D) Percentage of CFUs relative to untreated control. Cells were washed and placed in methylcellulose culture medium at the indicated time points after the addition of TDZD-8. (E) Loss of membrane integrity assessed by YoPro-1 uptake after 15 minutes of TDZD-8 treatment. Multispectral imaging flow cytometry shows the internalization of YoPro-1. Cells were stained with CD45 to delineate the plasma membrane and with the cell-permeable DNA dye Draq5 to identify the nucleus. (F) Flow cytometric histograms for YoPro-1 and PI overlaying TDZD-8–treated (20 μM for 15 minutes) normal mononuclear cells or primary AML cells over untreated controls. Treated cells are represented with black line histograms and untreated controls with gray solid histograms. (G) Percent viability of primary AML cells pretreated with Z-VAD (□) for 1 hour before the treatment with TDZD-8 20 μM. Viability was determined 24 hours after the addition of TDZD-8. Error bars represent the SEM.
TDZD-8 induces cell death with extremely rapid cell death kinetics showing loss of membrane integrity. (A) Percent viability assessed at the indicated time points for CD34+CD38− populations of primary AML specimens (n = 8) treated with TDZD-8 (■) or PTL (□). Percent viability is represented relative to untreated control. (B) Percent viability assessed at the indicated time points for unfractionated primary AML specimens (n = 17) treated with TDZD-8. Percent viability is shown relative to untreated controls. Error bars represent SEM. (C) Cells were treated with 20 μM TDZD-8 for the indicated periods of time, then washed and placed in culture until analysis at 24 hours. Percent viability represented relative to untreated control. (D) Percentage of CFUs relative to untreated control. Cells were washed and placed in methylcellulose culture medium at the indicated time points after the addition of TDZD-8. (E) Loss of membrane integrity assessed by YoPro-1 uptake after 15 minutes of TDZD-8 treatment. Multispectral imaging flow cytometry shows the internalization of YoPro-1. Cells were stained with CD45 to delineate the plasma membrane and with the cell-permeable DNA dye Draq5 to identify the nucleus. (F) Flow cytometric histograms for YoPro-1 and PI overlaying TDZD-8–treated (20 μM for 15 minutes) normal mononuclear cells or primary AML cells over untreated controls. Treated cells are represented with black line histograms and untreated controls with gray solid histograms. (G) Percent viability of primary AML cells pretreated with Z-VAD (□) for 1 hour before the treatment with TDZD-8 20 μM. Viability was determined 24 hours after the addition of TDZD-8. Error bars represent the SEM.
The short exposure time for commitment to cell death suggests that TDZD-8 may be rapidly binding or internalized by cells. Because the hydrophobic chemical structure of TDZD-8 predicts that the drug is likely to intercalate in membranes, experiments were performed to analyze plasma membrane integrity. For this purpose, several nucleic acid dyes of varying sizes were used (YoPro-1, Hoescht-33342, 7-AAD, and PI). The uptake of smaller dyes, YoPro-1 and Hoescht-33342, can be altered by relatively moderate changes in membrane permeability, whereas larger dyes such as 7-AAD and PI are only internalized when profound loss of membrane integrity occurs. Figure 4E shows a representative example of a primary AML specimen treated with TDZD-8 for 15 minutes and analyzed by nucleic acid dye labeling and multispectral imaging flow cytometry (Amnis Imagestream). The left panels show dye uptake analysis in control or TDZD-8–treated cells in which R1 represents intact cells (impermeable to YoPro-1), R2 shows cells with compromised membrane integrity (YoPro-1 permeable), and R3 represents dead cells (permeable to YoPro-1 and 7-AAD). The percentage of YoPro-1–positive cells (R2) increased from 7% to 78% upon treatment with TDZD-8 for 15 minutes. In addition, cells were also labeled with anti-CD45 to delineate the plasma membrane and the cell-permeable DNA dye Draq5 to identify the nucleus. Figure 4E (right panel) shows representative pictures of cells present in the R1, R2, and R3 gates, in which the nuclear localization of YoPro-1 is evident in R2 but not in R1. Additional studies in Figure 4F show that the rapid uptake of YoPro-1 observed for primary AML cells is not evident in normal specimens (BM or CB). These data indicate that TDZD-8 mediates a rapid alteration in membrane permeability in primary AML cells but not in normal hematopoietic cells.
With respect to the death mechanism, TDZD-8–treated cells show a rapid increase in Annexin V labeling, a marker of apoptosis (data not shown). Because loss of membrane integrity and Annexin V binding are early events associated with apoptosis, we tested downstream events involved in caspase-dependent apoptotic events (eg, pro-caspase and PARP cleavage). Interestingly, no cleavage of pro-caspases 3 or 8 or PARP was detected (data not shown). Moreover, no abrogation of death was observed upon treatment with the pan-caspase inhibitor Z-VAD (Figure 4G, □). These data suggest that death occurs by a caspase-independent pathway.
TDZD-8 activity as a kinase inhibitor
TDZD-8 has been reported to be a GSK-3β kinase inhibitor (IC50 = 2 μM) and to not significantly affect the activities of Cdk-1/cyclin B, CK-II, PKA, and PKC (IC50 > 100 μM).24 Therefore, to determine whether the antileukemia activity observed with TDZD-8 involves GSK3β inhibition, we tested other commercially available GSK3β inhibitors. Seven of the 8 agents tested failed to induce AML cell death (Table 2). The single other GSK3β inhibitor [2-chloro-1-(4,5-dibromo-thiophen-2-yl-ethanone)] that could induce AML cell death was also toxic to normal cells. In addition, we tested different compounds that share the thiazolidinedione ring structure and did not observe the AML specificity or cell death kinetics obtained with TDZD-8 (Table 2). Because the concentrations that induce leukemia-specific cell death are 10 times higher than the IC50 reported for inhibition of GSK3β, it is likely that the TDZD-8 antileukemia activity is due to an off-target effect. To test this hypothesis, a commercial kinase profiling service was used to identify other potential targets of TDZD-8. A broad range of 44 different transmembrane and intracellular kinases was examined at a drug concentration of 20 μM. Greater than 90% inhibition was observed for 14 different classes of kinase. Of those, the most evident enzymes with potential ties to hematologic malignancy were PKC and FLT3. Notably, other related kinases implicated in hematologic diseases (c-kit, PDGF-R, Jak2, Src, and Tie2) showed little to no inhibition.
Inhibitors tested on primary AML and normal specimens
| Activity . | Compound . | Cytotoxic . | Rapid kinetics . | |
|---|---|---|---|---|
| AML . | Normal . | |||
| GSK-3β inhibitor | TDZD-8 (4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione) | Yes | No | Yes |
| GSK-3β inhibitor | BIO | No | No | No |
| GSK-3β inhibitor | (5-Methyl-1H-pyrazol-3-yl)-(2-phenylquinazolin-4-yl)amine | No | No | No |
| GSK-3β inhibitor | 2-Chloro-1-(4,5-dibromo-thiophen-2-yl)-ethanone | Yes | Yes | No |
| GSK-3β inhibitor | TWS119 | No | No | No |
| GSK-3β inhibitor | SB-216763 | No | No | No |
| GSK-3β inhibitor | AR-A014418 | No | No | No |
| GSK-3β inhibitor | 1-Azakenpaullone | No | No | No |
| GSK-3β inhibitor | 2,4-Dibenzyl-5-oxothiadiazolidine-3-thione | No | No | No |
| Thiazolidine ring | Anthrax Lethal Factor protease inhibitor | No | No | No |
| (thiazolidinedione ring) | 2,4-thiazolidinedione | No | No | No |
| (Thiazolidinedione ring) PPARα/γ agonist | DRF 2519 | No | No | No |
| (Thiazolidinedione ring) PPARα/γ agonist | Troglitazone | Yes | Yes | No |
| (Thiazolidinedione ring) Erk inhibitor | -(2-Aminoethyl)-5-((4-ethoxyphenyl)methylene)-2,4-thiazolidinedione, HCl | No | No | No |
| (Thiazolidinedione ring) PI3-K inhibitor | 5-(2,2-Difluoro-benzo[1,3]dioxol-5-ylmethylene)-thiazolidine-2,4-dione | No | No | No |
| Activity . | Compound . | Cytotoxic . | Rapid kinetics . | |
|---|---|---|---|---|
| AML . | Normal . | |||
| GSK-3β inhibitor | TDZD-8 (4-Benzyl-2-methyl-1,2,4-thiadiazolidine-3,5-dione) | Yes | No | Yes |
| GSK-3β inhibitor | BIO | No | No | No |
| GSK-3β inhibitor | (5-Methyl-1H-pyrazol-3-yl)-(2-phenylquinazolin-4-yl)amine | No | No | No |
| GSK-3β inhibitor | 2-Chloro-1-(4,5-dibromo-thiophen-2-yl)-ethanone | Yes | Yes | No |
| GSK-3β inhibitor | TWS119 | No | No | No |
| GSK-3β inhibitor | SB-216763 | No | No | No |
| GSK-3β inhibitor | AR-A014418 | No | No | No |
| GSK-3β inhibitor | 1-Azakenpaullone | No | No | No |
| GSK-3β inhibitor | 2,4-Dibenzyl-5-oxothiadiazolidine-3-thione | No | No | No |
| Thiazolidine ring | Anthrax Lethal Factor protease inhibitor | No | No | No |
| (thiazolidinedione ring) | 2,4-thiazolidinedione | No | No | No |
| (Thiazolidinedione ring) PPARα/γ agonist | DRF 2519 | No | No | No |
| (Thiazolidinedione ring) PPARα/γ agonist | Troglitazone | Yes | Yes | No |
| (Thiazolidinedione ring) Erk inhibitor | -(2-Aminoethyl)-5-((4-ethoxyphenyl)methylene)-2,4-thiazolidinedione, HCl | No | No | No |
| (Thiazolidinedione ring) PI3-K inhibitor | 5-(2,2-Difluoro-benzo[1,3]dioxol-5-ylmethylene)-thiazolidine-2,4-dione | No | No | No |
To further examine PKC and FLT3, initial studies were performed to examine the activity of each in primary specimens and in response to drug treatment. First, because PKC was previously reported to not be a target of TDZD-8 (IC50 > 100 μM), we analyzed one family member from each of the 3 major PKC classes (conventional, novel, and atypical). The in vitro IC50 for PKC isoforms was PKCβI = 1.4 μM, PKCδ = 1.1 μM, and PKCι = 5.5 μM. Thus, at least in vitro, TDZD-8 appears to be a broad inhibitor of the PKC family. As a control for drug activity, GSK3β was also tested and, consistent with previous reports, had an IC50 of 1.4 μM. Next, to examine the activity of PKC in primary cells, immunoblots of purified CD34+ populations from AML and normal BM specimens were performed. As shown in Figure 5A, the levels of both total and phosphorylated-PKC were much higher in primary CD34+ AML specimens compared with normal controls, suggesting a role for the PKC family in primitive leukemic cells. Because active PKCs are localized in the plasma membrane40,41 and PKCα and PKCβ plasma membrane localization has been reported in leukemia cell lines,42 we examined their levels upon TDZD-8 treatment. Figure 5B shows that plasma membrane localized PKCα and PKCβ decreases with TDZD-8 exposure, suggesting that TDZD-8 induces PKC inactivation in primary CD34+ AML and ALL cells. We did not observe a difference in the levels of HSP70, suggesting that reduction of PKC in the membrane is not due to a general loss of membrane proteins. The data in Figure 5A and B indicate that the PKC family members are active in primary AML cells and that TDZD-8 potentially inhibits their function.
TDZD-8 inhibits PKC and FLT3 in primary AML specimens. (A) Immunoblots for CD34+ AML and normal BM specimens to determine PKC phosphorylation. Actin is shown as a loading control. (B) Primary CD34+ AML and ALL specimens treated with TDZD-8 for 1 hour were processed to obtain membrane fractions. Immunoblots were performed to determine PKCα and PKCβ levels in the membrane. HSP70 is shown as a control. (C) Titration curve and IC50 value for FLT3 kinase assay. (D) Overlays of flow cytometric analysis for phospho-FLT3 in primary AML specimens. Light gray solid line histograms represent untreated cells. Dotted line histograms represent TDZD-8–treated cells. Gray solid no line histogram represent controls. Cells were processed for analysis 30 minutes after the addition of drug. CD34+ (31% inhibition; left panel) and CD34+CD38− (29.5% inhibition; right panel) populations.
TDZD-8 inhibits PKC and FLT3 in primary AML specimens. (A) Immunoblots for CD34+ AML and normal BM specimens to determine PKC phosphorylation. Actin is shown as a loading control. (B) Primary CD34+ AML and ALL specimens treated with TDZD-8 for 1 hour were processed to obtain membrane fractions. Immunoblots were performed to determine PKCα and PKCβ levels in the membrane. HSP70 is shown as a control. (C) Titration curve and IC50 value for FLT3 kinase assay. (D) Overlays of flow cytometric analysis for phospho-FLT3 in primary AML specimens. Light gray solid line histograms represent untreated cells. Dotted line histograms represent TDZD-8–treated cells. Gray solid no line histogram represent controls. Cells were processed for analysis 30 minutes after the addition of drug. CD34+ (31% inhibition; left panel) and CD34+CD38− (29.5% inhibition; right panel) populations.
To examine the inhibitory effect of TDZD-8 on FLT3 activity, titration assays for FLT3 kinase activity were performed to estimate the in vitro IC50 of TDZD-8. Figure 5C shows the titration curve, which shows an IC50 of 673 nM in vitro. Next, to assess whether TDZD-8 inhibitory FLT3 activity could be detected in vivo, fluorescence-activated cell sorting analyses were performed to measure the phosphospecific (ie, activated) FLT3 form. As shown in Figure 5D, phosphorylated FLT3 is readily detected in primary AML specimens (as previously reported43,–45 ), and treatment with TDZD-8 induced an approximate 30% reduction in phosphorylation for both CD34+ and CD34+CD38− populations. These data suggest TDZD-8 may also function as an intracellular inhibitor of FLT3 activation.
Discussion
Identification of drugs that specifically target leukemia stem cells represents a significant challenge for several reasons. First, LSCs share many features in common with normal stem cells, including a largely quiescent cell-cycle status and the possible expression of membrane pumps that efflux many known drugs.46 Thus, by their nature, stem cells are refractory to most current forms of therapeutic challenge. Moreover, because normal and leukemic stem cells have similar properties, designing therapies that eradicate LSCs while sparing HSCs is likely to be difficult. Nonetheless, in recent years some unique features of LSCs have been described, and clear preclinical evidence now exists showing that LSCs are differentially susceptible to certain agents. Thus far, the molecular features strongly associated with LSC-specific cell death are (1) inhibition of NF-κB (and/or other survival factors such as Akt) and (2) induction of oxidative stress.47 At least 3 distinct regimens have been reported that fulfill these criteria and show selective targeting of LSCs.14,22,48 Notably, each approach described induces cell death within a time frame of approximately 12 to 18 hours, a rate we had previously considered relatively fast.
In the present study we describe unique antileukemia properties of the compound TDZD-8, which was originally developed as a non-ATP competitive inhibitor of GSK3β. The compound has undergone preclinical analysis as a cytoprotective agent in numerous models, including studies of type 2 diabetes, Alzheimer disease, spinal cord injury, and several forms of inflammation. Our findings add an entirely new dimension to the activities of TDZD-8 by showing that the compound selectively induces death of several major forms of leukemia cells, including malignant myeloid stem and progenitor populations, while sparing normal hematopoietic tissue. Further, the rate of cell death is exceptionally fast, with most toxicity evident within 1 to 6 hours. Because of these data, we believe that TDZD-8 may function by a novel mechanism to induce death of malignant hematopoietic cells.
Another striking feature of TDZD-8 is its apparent affinity to target cells. Experiments showed that exposure to the drug of only 30 minutes was sufficient to mediate all cytotoxic activity. Additional preliminary data indicate that treatments as brief as 5 minutes may also be effective (data not shown). Given the hydrophobic nature of TDZD-8, we speculate that the molecule is rapidly inserted into cellular membranes. This premise in turn led us to hypothesize that one component of the drug's mechanism might be a direct effect on the plasma membrane. Subsequent studies confirmed that TDZD-8 induces a rapid change in membrane integrity, such that small nucleic acid dyes are readily internalized. These findings support the concept that TDZD-8 directly modulates membrane integrity, and we propose that this activity is at least one component of the remarkable kinetics of cell death observed for the compound. Interestingly, if TDZD-8 does indeed function by plasma membrane alterations, it strongly suggests that malignant hematopoietic cells must possess unique features that confer their differential sensitivity. Although the specific molecular properties that distinguish leukemic from normal cell membranes are largely uncharacterized, we suggest that TDZD-8 may be a powerful tool in elucidating key changes that occur as part of the neoplastic transformation process.
Aside from the novel membrane-directed biology described earlier, TDZD-8 also functions as a multikinase inhibitor. Among those targets is, as described, GSK3β.24 Notably, analysis of several other known GSK3β inhibitors failed to induce leukemia-specific cell death (Table 2), suggesting that the activity of TDZD-8 is independent of GSK3β or at least combined with other activities. Other targets of TDZD-8, both in vitro and in vivo, are PKC family members and FLT3. PKC is known to play a role in growth and differentiation of hematopoietic cells.42 Moreover, it has been reported that PKCα overexpression confers chemoresistance to leukemia cells and is associated with poor survival,49,50 and that PKCβ is important for differentiation of HL-60 and U937 cells.51,52 In addition, PKCι is involved in protection of K562 cells against drug-induced apoptosis.53 These findings support a role for PKC in malignant hematopoiesis and suggest that the PKC family may represent an important target for therapy. Similarly, aberrant activation of FLT3 is a well-described feature of AML cell types, and inhibition of FLT3 clearly mediates an antileukemic effect in several systems.44,54 Thus, the activity of TDZD-8 toward FLT3 and PKC are potentially important components of its overall mechanism of action.
Finally, we note that previous studies have described TDZD-8 as a moderate NF-κB inhibitor,27,–29,32 an activity we have confirmed in leukemia cells (data not shown). Further, our data indicate that the compound is a strong oxidant (Figure 3). Therefore, TDZD-8 fulfills the 2 criteria described earlier that have previously been reported for regimens that selectively target LSCs (ie, inhibition of NF-κB and induction of oxidative stress).47 However, as shown in Figure 4, in addition to the established mechanisms of LSC death induction, TDZD-8 also confers a rapid alteration in membrane permeability for malignant cells of hematologic origin. Thus, we propose that loss of membrane integrity substantially accelerates the rate of cell death and is integral to the overall effects observed for TDZD-8. Whether the kinase inhibitory activity (Figure 5) further contributes to leukemic cell death is as yet unknown, but it remains an intriguing potential component of the overall activity of TDZD-8. To our knowledge, no other known compound combines the mechanism previously described to induce cell death, particularly in a fashion that is selective to malignant cell types.
In conclusion, our data show that the effect of TDZD-8 on primary leukemia cells is unique from several perspectives. First, the cytotoxicity is highly specific to leukemic cells and is not observed for normal hematopoietic cells or other tumors types. Second, the cell death kinetics are extremely fast, averaging only 2 hours. Third, irreversible commitment to cell death is also rapid, occurring in 30 minutes or less. Fourth, and perhaps most importantly, the cytotoxic activity of TDZD-8 is as potent for myeloid leukemic stem and progenitor cells as is it for bulk blast populations from both lymphoid and myeloid malignancies. Therefore, we propose that (1) TDZD-8 should be further characterized as a potential therapeutic agent and (2) it represents a powerful tool to elucidate key properties of leukemia cells that are distinct from normal hematopoietic cell types.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs James Palis, Michael W. Becker, and Duane C. Hassane for critical evaluation of the manuscript.
This work was supported by the Douglas Kroll research foundation, the Leukemia and Lymphoma Society (6099-06), the National Cancer Institute (R01CA90446), and the James P. Wilmot Cancer Center.
This manuscript is dedicated in loving memory of Fernando José Guzmán España.
C.T.J. is a scholar of the Leukemia and Lymphoma Society.
Authorship
Contribution: M.L.G. designed the research, analyzed data, conducted experiments, and wrote the manuscript; X.L., C.A.C., and R.M.R. conducted experiments; T.B. conducted experiments and analyzed data; J.L.L. and J.H. contributed vital reagents; F.Y. contributed vital reagents and analyzed data; and C.T.J. designed the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Monica L. Guzman, University of Rochester Medical Center, 601 Elmwood Ave Box 703, Rochester, NY 14642; e-mail:monica_guzman@urmc.rochester.edu.

![Figure 1. TDZD-8 specifically induces cell death of primary leukemia specimens. (A) Primary AML (n = 37), CLL (n = 12), ALL (n = 6), bcCML (n = 6), and normal mononuclear cells (n = 13, obtained from BM [n = 3], CB [n = 7], or MPB [n = 3]) were cultured for 18 to 24 hours in the presence of 20 μM TDZD-8. Cell viability was assessed by Annexin V/7-AAD staining. Percent viability is represented relative to untreated control. Leukemia specimens were significantly (P < .001) more sensitive to TDZD-8 than were normal specimens. Error bars represent the SEM. All assays were performed in triplicate. (B) Representative dot plots for the 24-hour flow cytometric analysis with Annexin V/7-AAD stain in the presence or the absence of 20 μM TDZD-8.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-05-088815/3/m_zh80010810670001.jpeg?Expires=1769132385&Signature=UYXus4IWRgJYSPx7fKUXD~~59O7UkhoNxG0qwfikR~-WpcJKCMUOqqpNx-ucOKHSsVm4lftG9-oCbm8WWi2igoQA8oegCIvPviny7fFbYAcQNsn0fuWPtW5oHpWYO4X0rYiqAZsS4wYsSfc9fGZIYzUeZi5dCZ8qlOE8U~WqjOQ6thKvlSDAk8Qe-7FyWe3RTapqFuUW7r64jkXScozsMscAtXuNfSIo9dY5LjunCaHrC195Kf3lc8IlCWQEGuQwdaklc6ipfALZoehCvuphtknBZUNQ5yhmeb3yWkm7IqBzLmNpPHSn1uc9fx~ES8lTyi6d1uQACkW94nSkO78c3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal