The chemokine receptor CCR7 has been implicated in maintenance of thymus morphology and establishment of tolerance to self-antigens. In this study, we provide direct evidence that negative selection of maturing thymocytes is defective in CCR7-deficent mice. Impaired negative selection was observed after TCR/CD3 complex stimulation in vivo as well as in vitro and was prominent in both double-positive and semimature single positive cells (CD4+CD8−CD24high). It is noteworthy that thymocytes of CCR7−/− mice display defective negative selection in response to endogenous superantigens, demonstrating that the defect also occurs under physiological conditions. Disturbed negative selection was correlated with delayed activation kinetics and decreased calcium flux response of CCR7−/− thymocytes after in vitro TCR/CD3 stimulation, suggesting that an impaired response of CCR7−/− thymocytes via TCR-mediated signaling is responsible for defective negative selection in these mice.
Introduction
CCR7 is a major regulator of the immune system, orchestrating a broad spectrum of fundamental processes ranging from lymphoid organ development to induction of defensive immune responses as well as oral tolerance.1,–3 An important role for CCR7 in thymus compartmentalization and coordination of migratory events during thymopoiesis has been recently demonstrated.4,5 Mice deficient in CCR7 or in its ligands CCL19 and CCL21-ser (plt/plt mice6,7 ) display altered thymus architecture, impaired T-cell development, and decreased numbers of thymocytes.4,5 Alterations of the thymus architecture are frequently observed in mice suffering from autoimmunity, such as mice deficient in the transcriptional regulator autoimmune regulator (Aire) and nonobese diabetic (NOD) mice.8,9 In these strains, autoimmunity correlates with breakdown of central tolerance, although in NOD mice peripheral tolerance seems to be affected as well.10,–12 It has been noted previously that lack of CCR7 signaling is associated with the manifestation of autoimmunity to lachrymal and salivary glands.13 Expanding this view, we recently demonstrated that CCR7-deficient mice in fact display a more generalized autoimmune phenotype affecting several organs.14 These observations raised the possibility that CCR7-deficiency affects induction of central tolerance
The hallmark of central tolerance is the negative selection of potentially self-reactive thymocytes. This process guarantees the generation of a self-tolerant peripheral T-cell repertoire. The major mechanism of negative selection is clonal deletion. During this process, thymocytes reacting strongly to self-peptide–self-MHC complexes are eliminated.15,16 Several studies using well characterized in vivo and in vitro models of negative selection suggest that clonal deletion can occur in all developmental stages of thymocytes, including double-negative, double-positive (DP), and single-positive (SP).15,17,,–20 However, it is widely accepted that (for thymocytes expressing an MHC class II-restricted T-cell receptor, at least) deletion of autoreactive T cells occurs predominantly at a semimature SP stage of development characterized by high expression levels of CD24. CD4+CD8−CD24high cells are considered to be semimature because they become apoptotic in response to T-cell receptor (TCR) stimulation, whereas mature CD4+CD8−CD24low cells proliferate after such stimulus.15,21,22
In the present study, we demonstrate that negative selection of CD4+CD8−CD24high and DP thymocytes is strongly impaired in CCR7-deficient mice after in vivo TCR/CD3 complex stimulation. Thymocytes from CCR7−/− mice also resist negative selection occurring physiologically in response to endogenous superantigens (SAg). Impaired negative selection was also observed in vitro and was accompanied by delayed activation kinetics and decreased calcium flux response of DP and CD4+ SP CCR7-deficient thymocytes to TCR/CD3 complex ligation. Therefore, our data indicate that CCR7 deficiency lowers the sensitivity and, thereby, the reactivity of thymocytes to TCR-triggered signaling at distinct developmental stages.
Materials and methods
Mice
The generation of CCR7−/− mice has been described elsewhere.1 CCR7-deficient mice were backcrossed for at least 7 generations either to the C57BL/6 or to the BALB/c genetic background. In vitro stimulation with the OVA323-339 peptide was performed using thymocytes from DO11.10/CCR7+/+ and DO11.10/CCR7−/− mice. The animals were bred and maintained under specific pathogen-free conditions in Hannover Medical School's animal facility. Wild-type C57BL/6 CD45.2, congenic CD45.1, and BALB/c mice were either bred in the same facility or obtained from Charles River Laboratories (Sulzfeld, Germany). Mice were used at the age of 6 to 9 weeks. All animal experiments were performed in accordance with institutional guidelines after approval by the local committees.
Immunohistology
Mice were killed by CO2 inhalation. Thymi were excised, embedded in optimal cutting temperature compound, and frozen on dry ice. Cryosections (8 μm) were prepared, air-dried, and fixed in ice-cold acetone. Sections were blocked with 10% mouse or rat serum and stained with antibodies against the indicated markers. The following monoclonal antibodies (mAb) and reagents were used: custom-made anti-CD8β (RmCD8-2) followed by mouse anti–rat IgG-Cy3 (Jackson ImmunoResearch Laboratories, West Grove, PA); biotinylated anti-CD4 (RM4-4) followed by streptavidin-Alexa Fluor 488 (Invitrogen, Carlsbad, CA); unlabeled anti–Ep-CAM1 (G8.8) (BD Biosciences, San Jose, CA) followed by mouse anti–rat IgG-Cy3 and biotinylated Ulex europaeus agglutinin-1 (UEA-1; Vector Laboratories, Burlingame, CA) followed by streptavidin-Cy5 (Invitrogen). Images were acquired using either an Olympus BX61 microscope (Olympus, Hamburg, Germany) with an F-View II Camera and cellP software (Soft Imaging System, Münster, Germany) or a Zeiss Axiovert M200 microscope with Hamamatsu/Orca ER Camera and Axiovision 3.1 software (Carl Zeiss MicroImaging, Oberkochen, Germany). Medullary areas were marked and measured using the cellP software. Cells within the marked areas were counted using the ImageJ software (http://rsb.info.nih.gov/ij/).
Flow cytometry
Single-cell suspensions of thymi were obtained by mincing the organs through a nylon mesh. The following antibodies were used: custom-made anti-CD3 (17A2), anti-CD4 (RmCD4-2), and anti-CD8β (RmCD8-2); anti-CD5 (53-7.3), anti-CD8α (53-6.7), anti-CD24 (M1/69), anti-CD45.2 (104), anti-CD62-L (MEL-14), anti-CD69 (H1.2F3), anti-Vβ3 (KJ25), anti-Vβ5 (MR9-4), anti-Vβ11 (RR3–15), and anti-Vβ12 (MR11-1) (BD Biosciences). CCR7 staining was performed using a phycoerythrin-labeled monoclonal antibody (4B12; eBioscience, San Diego, CA). The stain was performed at 37°C as recommended by the manufacturer. For the experiments using DO11.10 mice, thymocytes were additionally stained with the TCR-specific antibody KJ1.26 (Invitrogen). Flow cytometry was performed either on a FACSCalibur or on an LSRII flow cytometer (BD Biosciences).
In vitro stimulation of thymocytes
Freshly isolated thymocytes (3.0 × 106) from DO11.10/CCR7+/+ or DO11.10/CCR7−/− mice were cultured in RPMI medium (Invitrogen) supplemented with 2-mercaptoethanol, l-glutamine, and 10% fetal calf serum (FCS) in the presence of either nonloaded or ovalbumin (OVA)-loaded, bone marrow–derived dendritic cells (DCs) (5 × 105). After 18 hours, cells were collected and analyzed by flow cytometry. DCs were prepared as described previously.23 In brief, 2 × 106 bone marrow cells from tibias and femurs of BALB/c mice were cultured in RPMI, 10% FCS, 2-mercaptoethanol, glutamine, and penicillin/streptomycin supplemented with 100-200 ng/mL granulocyte macrophage–colony-stimulating factor for 7 or 8 days. Cells were then stimulated for 18 hours with 1 μg/mL lipopolysaccharide to induce maturation and then loaded with OVA323-339 peptide for 2 hours before coincubation with thymocytes. Otherwise, non–TCR-transgenic thymocytes were cultured for 2, 6, 8, 12, or 24 hours in plates precoated with 10 μg/mL anti-CD3 antibody (17A2) in medium containing 10 μg/mL anti-CD28 antibody (37N).
Calcium flux
Thymocytes (2.5 × 107) were loaded with 4 μg/mL Fluo-4 and 10 μg/mL Fura Red in the presence of 0.02% Pluronic (all from Invitrogen) in 2.5 mL of phenol red-free RPMI medium containing 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 10% FCS for 30 minutes at 37°C. Cells were washed and labeled with rat-anti–mouse CD4-Cy5 and rat-anti–mouse CD8-APC-Cy7 for 15 minutes on ice. After washing, thymocytes were resuspended in 1 mL of medium containing 2 mM CaCl2 and incubated in the dark for 90 minutes at room temperature. Calcium influx was measured by flow cytometry at 37°C using a custom-built heating device. After measurement of baseline levels for 30 seconds, 5 μg rat-anti–mouse CD3 (17A2) plus 24 μg mouse-anti–rat IgG (Jackson ImmunoResearch) as cross-linking antibody were added. Events were collected for a total time of 5 minutes. As a positive control, calcium ionophor A23187 (Sigma) was added at a final concentration of 20 μg/mL. FlowJo software (Treestar, Ashland, OR) was used to analyze the calcium flux kinetics of CD4+ SP and CD4+CD8+ DP thymocytes.
Stable Bone Marrow Chimeras
Bone marrow from C57BL/6 CCR7-deficient (CD45.2) and congenic wild-type (CD45.1) female mice were prepared as described previously4 and mixed at a ratio of 1:1. A total of 1.0 × 107 cells were transferred into CD45.1 recipients. Before transplantation, recipients were irradiated (5.5 Gy) twice at an interval of 5 hours. Three months later, chimeric mice were killed, and thymi were harvested and analyzed by flow cytometry.
Statistical Analysis
Statistical analysis was accomplished using Prism 3 (GraphPad Software, San Diego, CA). Data were analyzed as indicated in the figure legends. P values less than or equal to .05 were considered significant.
Results
CCR7-deficient thymocytes displayed impaired negative selection after in vivo TCR/CD3 complex ligation
The presence of autoimmunity in CCR7−/− and plt/plt mice13,14 suggests that central tolerance is altered in these mice. To address this possibility, we induced negative selection of thymocytes by injecting wild-type and mutant mice with anti-CD3 mAb. In this model of negative selection, depletion of DP as well as semimature CD4+CD8−CD24high thymocytes is triggered experimentally in vivo as a consequence of TCR/CD3 complex stimulation.12,21,22 Elimination of DP cells is thought to be largely influenced by corticosteroids and/or inflammatory cytokines produced in response to the activation of peripheral T cells.20,24 In contrast, depletion of CD4+CD8−CD24high cells is believed to represent clonal deletion of cells undergoing negative selection in the medulla.18,20,22
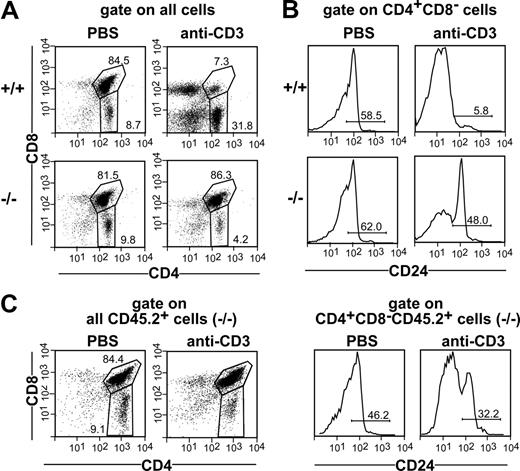
Negative selection was induced in wild-type and mutant mice by intravenously injecting 200 μg anti-CD3 mAb. Treated animals were killed 42 hours later, and thymocytes were analyzed for expression of CD4, CD8 and CD24 by flow cytometry. No differences between CCR7−/− and wild-type mice regarding the proportions of DP and SP thymocytes were observed after phosphate-buffered saline (PBS) injection, whereas injection of 200 μg anti-CD3 mAb induced depletion of the majority of wild-type but not of mutant DP thymocytes (Figure 1A). The percentage of cells belonging to the semimature CD4+CD8−CD24high subpopulation was also similar in wild-type and CCR7-deficient animals receiving PBS. However, whereas the administration of 200 μg anti-CD3 mAb induced the elimination of the majority of CD4+CD8−CD24high thymocytes in wild-type mice, only a slight reduction in this population was observed in CCR7−/− mice (Figure 1B).
Impaired negative selection of CCR7-deficient thymocytes in vivo. Nine-week-old C57BL/6 (+/+) and CCR7−/− (−/−) mice were intravenously injected with PBS or 200 μg of anti-CD3 mAb. Forty-two hours after injection, thymocytes were quantified, stained with antibodies to CD3, CD4, CD24, and CD8, and analyzed by flow cytometry. CD24 expression was analyzed to identify the semimature CD4+CD8−CD24high population undergoing negative selection after TCR ligation. (A) Proportions of CD4 SP and DP cells in the thymus of mice injected with PBS and mice injected with anti-CD3 mAb, demonstrating the depletion of the DP population in wild-type but not in mutant animals. (B) Proportions of CD4+CD8−CD24high cells in the thymi of mice injected with anti-CD3 mAb. Note that in contrast to wild-type mice, only a minority of CD4+CD8−CD24high cells have been depleted in CCR7-deficient animals after antibody administration. (C) Bone marrow of CD45.2 CCR7−/− mice was mixed with bone marrow of CD45.1 wild-type mice at ratio of 1:1 and transferred into irradiated CD45.1 wild-type recipients. Lack of depletion of CCR7-deficient DP and CD4+CD8−CD24high cells in a wild-type thymus environment indicates that an intrinsic defect in these thymocytes is responsible for the resistance of these cells to undergo depletion after TCR stimulation.
Impaired negative selection of CCR7-deficient thymocytes in vivo. Nine-week-old C57BL/6 (+/+) and CCR7−/− (−/−) mice were intravenously injected with PBS or 200 μg of anti-CD3 mAb. Forty-two hours after injection, thymocytes were quantified, stained with antibodies to CD3, CD4, CD24, and CD8, and analyzed by flow cytometry. CD24 expression was analyzed to identify the semimature CD4+CD8−CD24high population undergoing negative selection after TCR ligation. (A) Proportions of CD4 SP and DP cells in the thymus of mice injected with PBS and mice injected with anti-CD3 mAb, demonstrating the depletion of the DP population in wild-type but not in mutant animals. (B) Proportions of CD4+CD8−CD24high cells in the thymi of mice injected with anti-CD3 mAb. Note that in contrast to wild-type mice, only a minority of CD4+CD8−CD24high cells have been depleted in CCR7-deficient animals after antibody administration. (C) Bone marrow of CD45.2 CCR7−/− mice was mixed with bone marrow of CD45.1 wild-type mice at ratio of 1:1 and transferred into irradiated CD45.1 wild-type recipients. Lack of depletion of CCR7-deficient DP and CD4+CD8−CD24high cells in a wild-type thymus environment indicates that an intrinsic defect in these thymocytes is responsible for the resistance of these cells to undergo depletion after TCR stimulation.
The model of negative selection applied in our study assumes that bystander antigen-presenting cells (APCs) provide costimulation.21 Therefore, to exclude the possibility that impaired deletion of mutant thymocytes is caused by lack of proper costimulation by CCR7-deficient allophycocyanin, we generated chimeric animals displaying a chimerism of 30% to 50% CCR7−/− and 50% to 70% wild-type cells in the thymus. Chimeric animals were injected with either PBS or 200 μg anti-CD3 mAb and analyzed 42 hours later. As shown in Figure 1C, depletion of both DP and CD4+CD8−CD24high thymocytes of CCR7-deficient origin remained impaired even in the presence of wild-type APC. These results indicate that a cell-intrinsic defect is responsible for the resistance of mutant thymocytes to undergo deletion after TCR/CD3 complex stimulation.
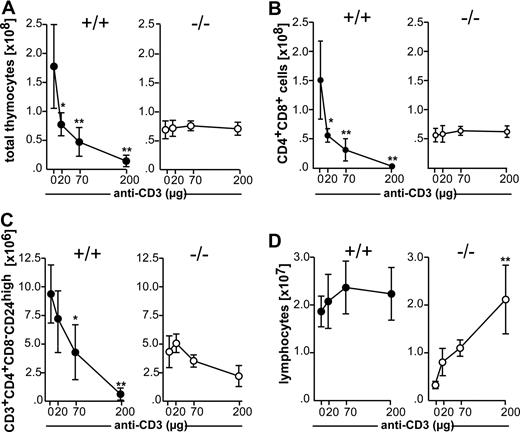
Negative selection of CD4+CD8−CD24high cells is Fas-independent after weak to moderate TCR ligation and Fas-dependent after strong TCR ligation.25 These conditions can be mimicked in vivo by injecting different concentrations of anti-CD3 mAb.12,20,25 Antibody at concentrations between 20 and 200 μg induced the elimination of thymocytes in wild-type mice in a dose-dependent manner. In marked contrast, no apparent reduction in the numbers of total thymocytes was observed in CCR7−/− mice (Figure 2A). The analysis of DP and semimature CD4+CD8−CD24high cells revealed that depletion of both populations was strongly impaired in CCR7-deficient animals at all doses of antibody administered (Figure 2B,C), indicating either that both Fas-dependent and Fas-independent pathways are affected in these mice or that the defect leading to the resistance to negative selection occurs upstream of both pathways. Lack of deletion of DP thymocytes in CCR7-deficient mice seems not to be due to the inability of peripheral T cells to respond to TCR stimulation, because lymphocytes derived from peripheral lymph nodes proliferate strongly in response to anti-CD3 injection (Figure 2D). The exaggerated proliferation of lymphocytes observed in peripheral lymph nodes of CCR7-deficient mice after anti-CD3 injection is a likely consequence of the T-cell lymphopenia found in the mutant organs under homeostatic conditions.1
Comparative analysis of thymocyte deletion in wild-type and CCR7-deficient mice after in vivo injection of anti-CD3 mAb. Nine-week-old C57BL/6 (+/+) and CCR7−/− (−/−) mice were intravenously injected with increasing doses of anti-CD3 mAb. Forty-two hours after injection, thymocytes were recovered and stained as described for Figure 1. (A) Total number of thymocytes. (B) Total number of DP thymocytes. (C) Total number of CD4+CD8−CD24high thymocytes. (D) Total number of lymphocytes isolated from peripheral lymph nodes in mice of both genotypes injected with PBS or different doses of anti-CD3 mAb, demonstrating a strong expansion of CCR7-deficient cells. Data shown was obtained from 12 mice of each genotype on C57BL/6 genetic background in 3 independent experiments. Similar results were also obtained for mutant mice on BALB/c background. Data has been subjected to 1-way ANOVA with Dunnett's multiple comparison post test. P values less than or equal to .05 were considered significant.
Comparative analysis of thymocyte deletion in wild-type and CCR7-deficient mice after in vivo injection of anti-CD3 mAb. Nine-week-old C57BL/6 (+/+) and CCR7−/− (−/−) mice were intravenously injected with increasing doses of anti-CD3 mAb. Forty-two hours after injection, thymocytes were recovered and stained as described for Figure 1. (A) Total number of thymocytes. (B) Total number of DP thymocytes. (C) Total number of CD4+CD8−CD24high thymocytes. (D) Total number of lymphocytes isolated from peripheral lymph nodes in mice of both genotypes injected with PBS or different doses of anti-CD3 mAb, demonstrating a strong expansion of CCR7-deficient cells. Data shown was obtained from 12 mice of each genotype on C57BL/6 genetic background in 3 independent experiments. Similar results were also obtained for mutant mice on BALB/c background. Data has been subjected to 1-way ANOVA with Dunnett's multiple comparison post test. P values less than or equal to .05 were considered significant.
Defective negative selection of CCR7-deficient thymocytes did not correlate with impaired migration of these cells into the medulla
It has been postulated recently that negative selection of thymocytes reactive to tissue-specific antigens should be impaired in the absence of CCR7-signaling due to the inability of positively selected cells to migrate from the cortex into the medulla, where negative selection would normally occur.13 However, this hypothesis cannot explain why the DP cell population of CCR7-deficient mice resists depletion after anti-CD3 injection, because these cells are virtually restricted to the cortex. Furthermore, only a minute fraction of DP thymocytes express CCR7 (1.4% of all DP cells; Figure 3A), indicating that the migratory behavior of DP cells toward CCR7-ligands is similar in wild-type and mutant mice. More importantly, less than one-fourth of positively selected DP cells (CD4+CD8+CD69+) express CCR7, suggesting that in addition to CCR7, other chemokine receptors might be involved in the transition of these cells from the cortex to the medulla (Figure 3A). CCR7 is largely expressed on SP cells (Figure 3A). It is noteworthy that CCR7 expression levels are variable within these populations, suggesting that the expression of the receptor is differentially regulated at different maturational stages. To test this hypothesis, we analyzed the expression of CCR7, CD24, and CD69 on CD4 SP cells concomitantly. We were surprised to find that CCR7 was either not expressed or expressed at low-to-intermediate levels on CD4+CD8−CD24high cells (Figure 3B subpopulations I and II). In contrast, the receptor was highly expressed on CD4+CD8−CD24int cells (Figure 3B subpopulation iii). This result indicates that CCR7 expression might be up-regulated after TCR stimulation on cells that escaped clonal deletion. Mature CD4+CD8−CD24low cells also express CCR7, although at intermediate levels (Figure 3B subpopulation iv). It is noteworthy that CCR7 expression levels seemed to correlate with the activation state of the cells, in that CD4+CD8−CD24int thymocytes express the highest levels of CD69 (Figure 3C subpopulation iii). Note that in a small fraction of CD4+CD8−CD24int cells, CD69 expression is down-regulated. It is likely that the decreased CD69 expression in these cells reflects their maturation into CD4+CD8−CD24low cells, which are CD69− (Figure 3C subpopulation iv).
CCR7 expression on different thymocyte subpopulations. Thymocytes of adult C57BL/6 mice were stained with anti-CD4, anti-CD8, anti-CD24, and anti-CD69 antibodies. CCR7 was detected using a monoclonal antibody. (A) CCR7 expression on DP and SP thymocytes. Populations were gated based on the expression of CD4, CD8, and CD69 as indicated. Bright gray indicates CCR7; dark gray, isotype control. Numbers indicate the percentage of cells expressing CCR7 above the background level. (B) CCR7 expression on CD4 SP cells at different maturational stages. The analysis of CCR7 and CD24 coexpression on CD4+CD8− cells allows the identification of 4 different subpopulations (i-iv). (C) Expression of CD69 on subpopulations i to iv depicted in panel B.
CCR7 expression on different thymocyte subpopulations. Thymocytes of adult C57BL/6 mice were stained with anti-CD4, anti-CD8, anti-CD24, and anti-CD69 antibodies. CCR7 was detected using a monoclonal antibody. (A) CCR7 expression on DP and SP thymocytes. Populations were gated based on the expression of CD4, CD8, and CD69 as indicated. Bright gray indicates CCR7; dark gray, isotype control. Numbers indicate the percentage of cells expressing CCR7 above the background level. (B) CCR7 expression on CD4 SP cells at different maturational stages. The analysis of CCR7 and CD24 coexpression on CD4+CD8− cells allows the identification of 4 different subpopulations (i-iv). (C) Expression of CD69 on subpopulations i to iv depicted in panel B.
Intriguingly, our data also demonstrate that CD4+CD8+CD69+CCR7+ cells express higher levels of CCR7 than CD4+CD8−CD24high cells. Therefore, either CCR7 is down-regulated on CD4+CD8+CD69+ thymocytes directly after positive selection or the CD4+CD8−CD24high cell population develops from positively selected DP thymocytes that do not express CCR7. In the latter case, no impairment in cortex-to-medulla migration of CD4 SP thymocytes would be expected in CCR7-deficient mice. One possibility to address this question is to analyze the distribution of SP cells in the thymus of CCR7-deficient mice. It has been previously reported that the medulla of sublethally irradiated CCR7-deficient mice contain strongly reduced numbers of SP thymocytes.5 Likewise, a reduction in the number of SP cells found in the medulla of nonirradiated CCR7−/−/AND-TCR transgenic mice was observed.5 Because nothing is known about the distribution of SP cells in the thymus of untreated CCR7−/− mice displaying a polyclonal TCR repertoire, we analyzed the presence of SP cells in the medulla of nonirradiated CCR7-deficient and wild-type mice. As shown in Figure 4A,B, many CD4+ and CD8+ SP cells were observed in the medulla of CCR7-deficient mice. It is noteworthy that even those medullary areas that were misplaced to the outer rim of the organ in the mutant mice were filled with SP cells (Figure 4A arrowheads). A quantitative evaluation of the medulla revealed that there was no significant difference in the number of CD4 SP cells found per area in wild-type and CCR7-deficient mice (121.9 ± 13.11 cells/0.01 mm2 vs 120.00 ± 13.47 cells/0.01 mm2, respectively; Figure 4C). In contrast, a 2.2-fold decrease in the number of medullary CD8 SP cells was observed (20.73 ± 8.62 cells/0.01 mm2 in wild-type vs 9.05 ± 2.19 cells/0.01 mm2 in mutants; Figure 4C). The reasons for the reduced number of medullary CD8 SP cells observed in CCR7-deficient animals are not known yet, but these cells may display a partial defect in cortex-to-medulla migration. Nevertheless, our data clearly indicate that the majority of the positively selected thymocytes of CCR7−/− animals migrate efficiently from the cortex into the medulla. Therefore, impaired negative selection of CCR7-deficient thymocytes was not causally correlated with impaired cortex-to-medulla migration.
Unimpaired migration of CD4+ cells in the medulla of CCR7-deficient mice. Cryosections of the thymi from adult (6- to 8-week-old) wild-type (+/+) and CCR7−/− (−/−) mice were stained with antibodies to CD4 (green) and CD8 (red) to identify DP and SP cells. DP cells appear in yellow. Nuclear staining was performed with 4,6-diamidino-2-phenylindole (DAPI; white). Cortex and medulla topography is reflected by the DAPI stain (left panels). CD4 SP cells are normally found in the medulla of CCR7-deficent animals. (A) A representative region (objective magnification, 4×; numeric aperture [NA], 0.16) is shown of the thymus of CCR7-deficient animals containing several medullary areas, all of them filled with CD4 SP cells (green). Note that even the medullary areas that are misplaced at the outer rim of the organ contain CD4 SP cells (arrows). (B) High magnification picture (objective magnification, 20×/0.75 NA) showing a representative medullary area of the thymus of wild-type (+/+) and CCR7-deficient mice (−/−). Whereas CD4 SP cells are normally found in the medulla of both mouse strains, CD8 SP cells are less abundant in CCR7−/− mice. Images were acquired using an Olympus BX61 microscope. See “Immunohistology” for more image acquisition information. (C) Mean (± SD) of the numbers of CD4 and CD8 SP cells per medullary area. Quantitative data were obtained from analysis of 3 mice from each genotype.
Unimpaired migration of CD4+ cells in the medulla of CCR7-deficient mice. Cryosections of the thymi from adult (6- to 8-week-old) wild-type (+/+) and CCR7−/− (−/−) mice were stained with antibodies to CD4 (green) and CD8 (red) to identify DP and SP cells. DP cells appear in yellow. Nuclear staining was performed with 4,6-diamidino-2-phenylindole (DAPI; white). Cortex and medulla topography is reflected by the DAPI stain (left panels). CD4 SP cells are normally found in the medulla of CCR7-deficent animals. (A) A representative region (objective magnification, 4×; numeric aperture [NA], 0.16) is shown of the thymus of CCR7-deficient animals containing several medullary areas, all of them filled with CD4 SP cells (green). Note that even the medullary areas that are misplaced at the outer rim of the organ contain CD4 SP cells (arrows). (B) High magnification picture (objective magnification, 20×/0.75 NA) showing a representative medullary area of the thymus of wild-type (+/+) and CCR7-deficient mice (−/−). Whereas CD4 SP cells are normally found in the medulla of both mouse strains, CD8 SP cells are less abundant in CCR7−/− mice. Images were acquired using an Olympus BX61 microscope. See “Immunohistology” for more image acquisition information. (C) Mean (± SD) of the numbers of CD4 and CD8 SP cells per medullary area. Quantitative data were obtained from analysis of 3 mice from each genotype.
CCR7−/− thymocytes displayed impaired negative selection in response to endogenous superantigens
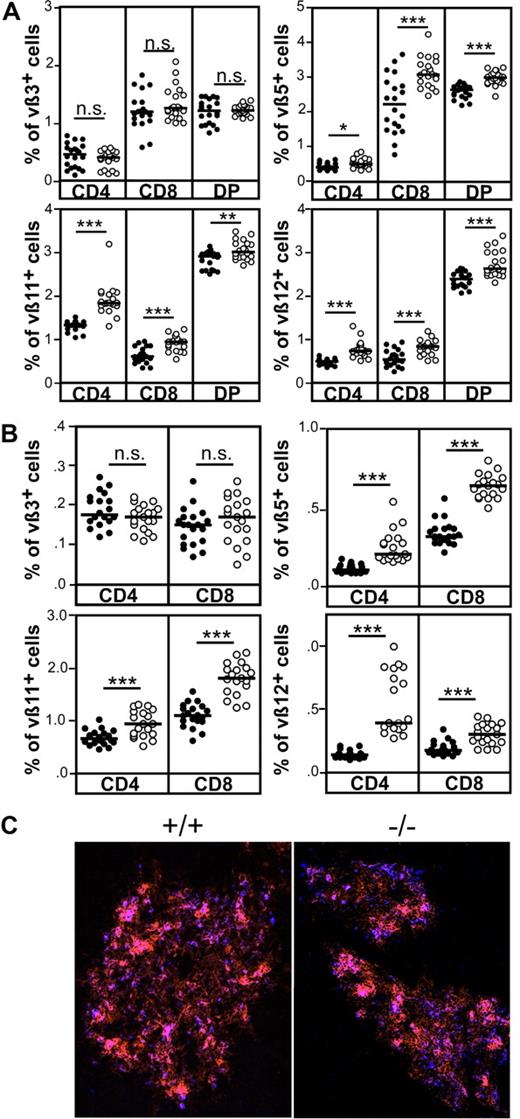
To investigate the capacity of CCR7-deficient thymocytes to undergo negative selection under physiological conditions, we compared the deletion efficiency of thymocytes reactive to endogenous retroviral SAg in wild-type and CCR7−/− animals. In this scenario, negative selection evokes clonal deletion of thymocytes bearing distinct TCR Vβ domains that react to SAg derived from mouse mammary tumor virus (Mtv). BALB/c mice are positive for Mtv-6, Mtv-8, and Mtv-9.26 Accordingly, thymocytes expressing Vβ3 (reactive to Mtv-6), Vβ5, Vβ11, and Vβ12 (reactive to Mtv-8 and Mtv-9) are deleted to a large extent in this mouse strain. As shown in Figure 5A, significantly higher percentages of DP and SP thymocytes expressing Vβ5, Vβ11, and Vβ12, but not Vβ3, were observed in CCR7-deficient mice. Similar results were also observed for splenocytes (Figure 5B). Together, the data indicate that negative selection of thymocytes reactive to Mtv-8 and Mtv-9 but not to Mtv-6 SAg is impaired in the absence of CCR7 signaling. At least for Vβ5+ thymocytes, clonal deletion is thought to be induced by a medullary thymic epithelial cell (mTEC) subpopulation that binds the lectin Ulex europaeus agglutinin-1 (UEA1).27 It has been described previously that clusters of mTEC expressing G8.8 and binding UEA-1 are missing in the thymus of CCR7-deficient mice.5 Therefore, the absence of such cell clusters may lead to impaired negative selection of thymocytes bearing the Vβ5 domain in CCR7-deficient animals. However, in contrast to the above- mentioned data, G8.8+/UEA-1+ mTEC clusters were present in CCR7-deficient animals (Figure 5C). These results demonstrate that impaired negative selection of Vβ5+ thymocytes is not caused by lack of G8.8+/UEA-1+ mTEC clusters in the medulla of CCR7−/− animals analyzed here.
Impaired negative selection of CCR7-deficient thymocytes in response to endogenous SAgs. (A) Negative selection of Vβ5+, Vβ11+, and Vβ12+, but not of Vβ3+, thymocytes is impaired in CCR7-deficient mice. (B) Increased proportions of Vβ5, Vβ11, and Vβ12 lymphocytes in the spleen of CCR7-deficient mice, indicating that autoreactive cells escaping negative selection in the thymus are not deleted in the periphery and, therefore, could induce the development of autoimmunity in these mice. Data has been subjected to unpaired T test. P values less than or equal to .05 were considered significant. (C) Clusters of G8.8+/UEA-1+ epithelial cells are present in the medulla of CCR7-deficient animals, demonstrating that impaired negative selection of thymocytes reactive to endogenous SAg is not due to the absence of cells able to induce their deletion. Cryosections of the thymus from wild-type and mutant mice were stained with antibodies to EpCAM1 (G8.8, red) as well as with UEA-1 (blue). Images were acquired using a Zeiss Axiovert M200 microscope. Objective magnification, 10×/0.45 NA.
Impaired negative selection of CCR7-deficient thymocytes in response to endogenous SAgs. (A) Negative selection of Vβ5+, Vβ11+, and Vβ12+, but not of Vβ3+, thymocytes is impaired in CCR7-deficient mice. (B) Increased proportions of Vβ5, Vβ11, and Vβ12 lymphocytes in the spleen of CCR7-deficient mice, indicating that autoreactive cells escaping negative selection in the thymus are not deleted in the periphery and, therefore, could induce the development of autoimmunity in these mice. Data has been subjected to unpaired T test. P values less than or equal to .05 were considered significant. (C) Clusters of G8.8+/UEA-1+ epithelial cells are present in the medulla of CCR7-deficient animals, demonstrating that impaired negative selection of thymocytes reactive to endogenous SAg is not due to the absence of cells able to induce their deletion. Cryosections of the thymus from wild-type and mutant mice were stained with antibodies to EpCAM1 (G8.8, red) as well as with UEA-1 (blue). Images were acquired using a Zeiss Axiovert M200 microscope. Objective magnification, 10×/0.45 NA.
Impaired responsiveness of CCR7-deficient thymocytes to TCR stimulation in vitro
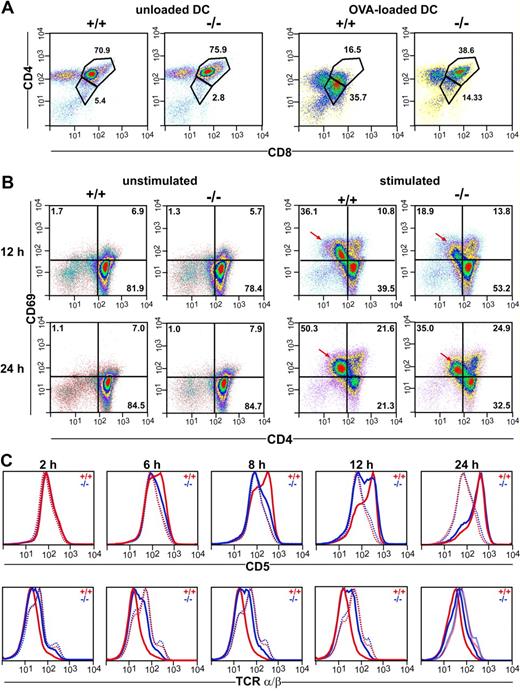
The observations described above would imply that thymocytes of CCR7−/− mice display a cell internal defect affecting their responsiveness to TCR stimulation. To test this hypothesis, we coincubated thymocytes of DO11.10/CCR7+/+ and DO11.10/CCR7−/− mice for 18 hours with mature bone marrow–derived DCs loaded with ovalbumin. As shown in Figure 6A, the majority of the DP thymocytes of DO11.10/CCR7+/+ mice become activated on TCR ligation. This is demonstrated by down-regulated expression of both CD4 and CD8 surface molecules.21 In contrast, a minor fraction of the DP thymocytes of DO11.10/CCR7−/− mice showed this behavior (Figure 6A). Similar results were also observed after in vitro stimulation of CCR7-deficient thymocytes with anti-CD3/anti-CD28 antibodies (data not shown). Together, these results support the idea that mutant thymocytes are more refractory to TCR stimulation than are wild-type thymocytes. To substantiate this idea, we stimulated thymocytes of both strains in vitro with anti-CD3/anti-CD28 antibodies and investigated the expression of surface markers, which are normally regulated on thymocytes subsequent to TCR activation. After a 12-hour stimulation period, we observed up-regulated expression of CD69 in twice as many wild-type thymocytes as in CCR7-mutant thymocytes (Figure 6B). Note that cells with increased CD69 expression had concurrent decreased CD4 surface levels (arrows in Figure 6B). In addition, whereas no differences in the expression levels of TCRα/β or CD5 were observed between nonstimulated wild-type and mutant thymocytes, the kinetic of CD5 up-regulation as well as of TCRα/β down-regulation was obviously delayed in thymocytes of CCR7−/− mice (Figure 6C). These results strongly support the hypothesis that the capacity of thymocytes to receive or transduce TCR-mediated signals is impaired in CCR7-deficent mice. Therefore, we subsequently examined the calcium flux responses in SP and DP thymocytes using a FACS-based single cell assay. For this purpose, thymocytes were labeled with the calcium indicators Fluo-4 and Fura Red and were stained with antibodies to CD4 and CD8. After CD3 cross-linking, calcium flux was measured over time and analyzed separately for the CD4+ SP and DP populations as indicated in Figure 7A. The calcium signal elicited by CD3 cross-linking was reduced in both CD4+ SP and DP CCR7-deficient thymocytes (Figure 7B). However, whereas the CCR7-deficient CD4+ SP thymocytes exhibited a reduced peak height of the calcium flux signal compared with wild-type cells (Figure 7B, red arrows), the calcium flux response of mutant DP thymocytes was reduced primarily because of a larger fraction of CCR7-deficient DP thymocytes remaining unresponsive to TCR stimulation (Figure 7B red arrowheads). In contrast, the response to the calcium ionophore A23187 was comparable for mutant and wild-type thymocytes (data not shown), indicating that the cellular mechanisms of calcium mobilization operate normally in CCR7-deficient mice.
Impaired responsiveness of CCR7-deficient thymocytes to TCR stimulation in vitro. (A) Thymocytes of DO11.10/CCR7+/+ (+/+) and DO11.10/CCR7−/− (−/−) mice were coincubated with either unloaded or OVA-loaded DCs. Cells were harvested after 18 hours of incubation and analyzed by flow cytometry. CCR7-deficient thymocytes were less responsive to TCR stimulation as indicated by the lower number of DP cells with decreased expression of CD4 and CD8 after stimulation. (B) Thymocytes of wild-type (+/+) and CCR7-deficient (−/−) mice were incubated for either 12 or 24 hours in the absence (unstimulated) or in the presence (stimulated) of antibodies to CD3 and CD28. Similar expression levels of CD69 were observed in unstimulated thymocytes of both strains. Although TCR stimulation induces up-regulation of CD69 on thymocytes of both strains, mutant thymocytes respond more slowly and less efficiently (→). For panels A and B, pseudocolor density plots are gated on living thymocytes according to forward and side scatter (FSC/SSC) profile, numbers indicate the percentage of cells in the specified regions. (C) Impaired responsiveness of CCR7-deficient thymocytes to CD3/CD28 sti-mulation is also reflected by delayed kinetic of CD5 up-regulation and TCR down-regulation. … indicates unstimulated; ——, stimulated. Data shown are representative of 3 independent experiments.
Impaired responsiveness of CCR7-deficient thymocytes to TCR stimulation in vitro. (A) Thymocytes of DO11.10/CCR7+/+ (+/+) and DO11.10/CCR7−/− (−/−) mice were coincubated with either unloaded or OVA-loaded DCs. Cells were harvested after 18 hours of incubation and analyzed by flow cytometry. CCR7-deficient thymocytes were less responsive to TCR stimulation as indicated by the lower number of DP cells with decreased expression of CD4 and CD8 after stimulation. (B) Thymocytes of wild-type (+/+) and CCR7-deficient (−/−) mice were incubated for either 12 or 24 hours in the absence (unstimulated) or in the presence (stimulated) of antibodies to CD3 and CD28. Similar expression levels of CD69 were observed in unstimulated thymocytes of both strains. Although TCR stimulation induces up-regulation of CD69 on thymocytes of both strains, mutant thymocytes respond more slowly and less efficiently (→). For panels A and B, pseudocolor density plots are gated on living thymocytes according to forward and side scatter (FSC/SSC) profile, numbers indicate the percentage of cells in the specified regions. (C) Impaired responsiveness of CCR7-deficient thymocytes to CD3/CD28 sti-mulation is also reflected by delayed kinetic of CD5 up-regulation and TCR down-regulation. … indicates unstimulated; ——, stimulated. Data shown are representative of 3 independent experiments.
Impaired calcium flux of CCR7-deficient thymocytes in response to CD3-ligation by cross-linking antibodies. (A) Wild-type (+/+) and CCR7-deficient (−/−) mice harbor comparable numbers of CD4+ SP and CD4+CD8+ DP thymocytes. Pseudocolor density plots are gated on living thymocytes according to forward and side scatter (FSC/SSC) profile. Numbers indicate the percentage of cells in the specified regions. (B) Impaired calcium flux of CCR7-deficient thymocytes in response to CD3 ligation by cross-linking antibodies. Pseudocolor density plots are gated on CD4+ SP or CD4+CD8+ DP thymocytes according to the gates depicted in (A). Black arrows indicate addition of CD3 and cross-linking antibodies. CCR7-deficient (−/−) CD4 SP thymocytes show reduced calcium flux in comparison to wild-type (+/+) as indicated by the red arrows. Note that a large fraction of the CCR7-deficient DP population remains mostly unresponsive (red arrowheads). Line graphs show median values of Fluo-4/Fura Red-ratio for wild-type (red) and CCR7-deficient thymocytes (blue). Data shown are representative of 3 independent experiments.
Impaired calcium flux of CCR7-deficient thymocytes in response to CD3-ligation by cross-linking antibodies. (A) Wild-type (+/+) and CCR7-deficient (−/−) mice harbor comparable numbers of CD4+ SP and CD4+CD8+ DP thymocytes. Pseudocolor density plots are gated on living thymocytes according to forward and side scatter (FSC/SSC) profile. Numbers indicate the percentage of cells in the specified regions. (B) Impaired calcium flux of CCR7-deficient thymocytes in response to CD3 ligation by cross-linking antibodies. Pseudocolor density plots are gated on CD4+ SP or CD4+CD8+ DP thymocytes according to the gates depicted in (A). Black arrows indicate addition of CD3 and cross-linking antibodies. CCR7-deficient (−/−) CD4 SP thymocytes show reduced calcium flux in comparison to wild-type (+/+) as indicated by the red arrows. Note that a large fraction of the CCR7-deficient DP population remains mostly unresponsive (red arrowheads). Line graphs show median values of Fluo-4/Fura Red-ratio for wild-type (red) and CCR7-deficient thymocytes (blue). Data shown are representative of 3 independent experiments.
Discussion
The presence of autoimmunity in CCR7-deficient mice13,14 indicates a failure in the induction of central tolerance in these animals. In the present study, we demonstrate that negative selection is in fact impaired in CCR7−/− mice. Hampered negative selection of CCR7-deficient thymocytes was observed in all in vivo and in vitro models we applied. We found elimination of DP as well as of semimature CD4+CD8−CD24high SP thymocytes induced by TCR/CD3 complex stimulation to be strongly impaired in CCR7-deficient mice. Although this model for induction of negative selection would be considered nonphysiological, our data demonstrate clearly that thymocytes of CCR7−/− mice do not respond to TCR/CD3 complex stimulation in the same way that wild-type thymocytes do. It is noteworthy that negative selection of thymocytes reactive to endogenous SAg also seems to be impaired in CCR7−/− animals because the extent of deletion of CCR7-deficient Vβ5+, Vβ11+, and Vβ12+ thymocytes was significantly decreased compared with control thymocytes. The proportions of Vβ5+, Vβ11+, and Vβ12+ thymocytes found in CCR7-deficient mice were within the range described in other mouse strains displaying defective negative selection. For example, similar proportions were reported in mice deficient in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or mice deficient in the costimulatory molecules B7-1 and B7-2.28,29 Remarkably, the degree of depletion of CCR7-deficient Vβ3+ thymocytes was normal, indicating that negative selection of thymocytes reactive to Mtv-8 and Mtv-9 but not reactive to Mtv-6 SAg was defective in CCR7−/− mice. Normal deletion frequencies of thymocytes expressing Vβ3 concomitantly with impaired depletion of thymocytes expressing Vβ5, Vβ11, and Vβ12 was also observed in Ikaros null mice and in CD28-deficient mice.26,28 It has been reported previously that BALB/c thymic DCs express Mtv-6 but not Mtv-8 and Mtv-9 SAg.30 This indicates that negative selection of Vβ3+ thymocytes, but not of Vβ5+, Vβ11+, and Vβ12+ thymocytes, may be accomplished by DCs. Therefore, the observed differences in deletion efficiency regarding thymocytes bearing distinct subsets of Vβ domains may reflect the cell type expressing the respective Mtv-Sag and thereby achieving negative selection. Indeed, because of their costimulatory capacities, DCs might induce clonal deletion more effectively and thus compensate for less vigorous TCR signals. This would explain the equivalent efficiency of DC-mediated depletion of Vβ3+ thymocytes in CCR7-deficient and wild-type mice. In contrast to the data presented here, it has been reported previously that negative selection of Vβ5+ thymocytes is not impaired in plt/plt mice, which are deficient in the CCR7 ligands CCL19 and CCL21-ser.5 The reasons for the discrepancy between our data and the data generated by others using plt/plt mice are currently unclear but may be attributed to the different mouse strains used in the studies. Thymocytes of plt/plt mice express CCR7 and CCL21-leu is present in some nonlymphoid organs of these mice. Therefore, it would be possible that this variant of CCL21 reaches the thymus via the blood stream and binds to stromal cells, thus compensating for the lack of CCL21-ser.
It has been proposed recently that negative selection of thymocytes reactive to tissue antigens is defective in the absence of CCR7-mediated signals as a result of impaired migration of positively selected cells from the cortex to the medulla.13 This hypothesis is based on the fact that reduced numbers of SP cells were observed in the medulla of irradiated CCR7−/− mice.5 However, although we observed a 2-fold reduction in the numbers of CD8 SP cells, we never observed reduced numbers of CD4 SP cells in the medulla of nonmanipulated CCR7-deficient mice. Therefore, differences in the applied experimental set-up are likely to generate the discrepancies in the results obtained in our study versus those reported previously. It has been demonstrated recently that sublethal irradiation leads to up-regulation of CCL25, CCL21, and CXCL12 mRNA expression by thymic dendritic and stromal cells.31 Therefore, the unbalanced chemokine-profile provoked by irradiation may influence the positioning of SP radio-resistant cells in the thymus depending on the presence or absence of CCR7. Likewise, the discrepancy observed between nonirradiated CCR7−/− bearing a polyclonal T cell repertoire and CCR7−/−/AND-TCR transgenic mice is likely to reflect differences between the analyzed mice. Ueno et al5 reported that the medullary regions were poorly formed in the thymus of CCR7−/−/AND-TCR mice, and many CD4 SP cells were found in the cortices of these animals. However, compared with non-TCR transgenic mice, AND-TCR transgenic mice display strongly skewed positive selection toward CD4 SP thymocytes.5,32 Moreover, the skewed positive selection of CD4 SP cells observed in these mice results in disruption of the thymus morphology and disorganization of the medullary epithelium.32 Therefore, the combination of the transgenic AND-TCR with CCR7 deficiency may create the unique phenotype observed by Ueno et al.5 Because our analysis of nonirradiated thymi of mice bearing a polyclonal TCR repertoire represents a physiological situation, we conclude that impaired cortex-to-medulla migration is not a major factor leading to defective negative selection of CCR7-deficient thymocytes. The finding that the elimination of DP cells, which reside in the cortex, was also defective in CCR7-deficient animals lends further support to this conclusion. In addition, only a minute fraction of CD4+CD8+ cells expresses CCR7. Therefore, it seems unlikely that this receptor is directly involved in the failure of these cells to undergo negative selection. The expression pattern of CCR7 on DP thymocytes observed in our present study is in agreement with data reported by others demonstrating that CCR7 mRNA expression by DP cells is extremely low and that these cells do not migrate in vitro in response to the CCR7-ligands CCL19 and CCL21.33,34 In addition, CCR7 was expressed on less than one-fourth of activated DP thymocytes (CD4+CD8+CD69+), which are thought to represent a positively selected population in transit between cortex and medulla. This result correlates very well with previously published data demonstrating that this cell population expresses low levels of CCR7 mRNA.34 In fact, it has been demonstrated by others that a similar fraction of these cells is able to migrate in vitro toward CCR7 ligands.33 It is noteworthy that CD4+CD8+CD69+ thymocytes migrate more efficiently to CCL22, CXCL12, and CCL25 than to CCL19 and CCL21.33 With the exception of CCL25, which is present in the whole thymus at high levels, the expression of the chemokines CCL17, CCL19, CCL21, CCL22, and CXCL12 is either more prominent or restricted to the medulla and corticomedullary junction.35 Therefore, chemokine receptors other than CCR7 might guide cortex-to-medulla migration of positively selected thymocytes.
As expected, CCR7 expression was detected in the majority of the CD4 SP cells of wild-type mice. It is noteworthy that the analysis of CCR7 and CD24 coexpression on these cells revealed that only part of the most immature CD4+CD8−CD24high subpopulation expressed the receptor. Furthermore, CCR7 is expressed on these cells at low to intermediate levels, whereas the more mature CD4+CD8−CD24int subpopulation express high levels of CCR7. This observation indicates that CCR7 expression positively correlates with the maturation of CD4 SP thymocytes, because the receptor seems to be up-regulated on thymocytes upon TCR stimulation. Expression of CCR7 on SP thymocytes that escaped negative selection could be necessary for thymocyte/medullary stroma cell interactions, thus assuring their proper functional maturation. Actually, our data demonstrate that CCR7 is not crucial for the migration of the majority of positively selected thymocytes into the medulla. A definitive confirmation of this hypothesis will require further experiments analyzing the migratory behavior of positively selected cells by 2-photon microscopy. Nevertheless, it seems unlikely that the function of CCR7 in negative selection and maturation of SP cells is restricted to cortex-to-medulla migration. The results of the in vitro experiments clearly demonstrate that thymocytes of CCR7-deficient mice are at least in part hyporesponsive to TCR ligation: (1) Only a small proportion of DP thymocytes of CCR7-deficient mice express decreased levels of CD4 and CD8 after stimulation with either OVA-peptide or anti-CD3 plus anti-CD28 antibodies. (2) Only a fraction of CCR7−/− thymocytes exhibit increased surface expression of CD69 after anti-CD3/anti-CD28 stimulation, indicating that most of those cells were not activated after TCR cross-linking. (3) Thymocytes of CCR7−/− mice show delayed up-regulation of CD5 as well as down-regulation of TCR α/β expression after in vitro stimulation. (4) Finally, DP as well as CD4 SP mutant thymocytes display reduced calcium flux responses after CD3 cross-linking.
Remarkably, CCR7 is virtually not expressed on DP cells. This observation suggests that the defect leading to impaired negative selection in CCR7-deficient animals occurs either at a developmental stage preceding the DP stage or is not directly related to CCR7 signaling on selecting thymocytes. The thymi of CCR7/CCR7-ligand-deficient mice display a strongly disrupted architecture, indicating that stromal cell/thymocyte cross-talk is impaired in these animals.4,5 Therefore, it seems possible that impaired thymic cross-talk during early developmental stages eventually results in the differentiation of thymocytes displaying an impaired capability to undergo activation. It is noteworthy that CCR7 deficiency could influence thymic cross-talk in different ways. One possibility is that stromal cells developing in the absence of CCR7-signaling are defective and unable to transduce the signals necessary for proper T-cell development during stromal cell/thymocyte interactions. In this case, thymic cross-talk would be impaired independently of the expression of CCR7 on thymocyte precursors. A further possibility would be that lack of CCR7 expression on thymocyte precursors directly hampers their migratory behavior and their capacity to meet stromal cells expressing CCL19/CCL21, thereby impeding stromal cell/thymocyte interactions. On the other hand, absence of CCR7 might influence the stability of stromal cell/thymocyte interactions, given that CCR7-mediated signals increase the avidity of integrins favoring the stabilization of cell-cell interactions.36
In addition, other mechanisms may contribute to the resistance of CCR7-deficient SP thymocytes to undergo negative selection. Surface-bound CCL19 and CCL21 have been demonstrated to capture and polarize CCR7-expressing T cells. This enhances T-cell capacity to stably interact with antigen-presenting cells, thus favoring immunological synapse formation.37 Lack of CCR7 on thymocytes undergoing negative selection may result in less stable interactions with medullary thymic epithelial and dendritic cells. These impaired interactions could further contribute to the decreased TCR activation observed in vivo in CCR7-deficient thymocytes. An additional factor influencing negative selection of CCR7-deficient thymocytes would be an impaired induction of activation-induced cell death after TCR stimulation. Indeed, recently published data indicate that this process should be impaired in the absence of CCR7-signaling.38
In summary, our present study indicates that decreased responsiveness of CCR7−/− thymocytes to TCR stimulation is a major factor leading to impaired negative selection in CCR7-deficient animals. In addition, our findings identify impaired negative selection as a factor contributing to the autoimmune phenotype observed in CCR7/CCR7-ligand-deficient animals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Oliver Pabst and Michael Morgan for critical reading of the manuscript.
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB587-B3 (R.F.).
Authorship
Contribution: A.C.M.D.-M. designed the study, performed research, analyzed data, and wrote the manuscript. T.W. performed research and analyzed data. S.W. performed research. G.B. wrote the manuscript. R.F. designed the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Dr Reinhold Förster, Institute of Immunology, Hannover Medical School, Carl-Neuberg-Str. 1 30625 Hannover, Germany; e-mail:foerster.reinhold@mh-hannover.de.


![Figure 4. Unimpaired migration of CD4+ cells in the medulla of CCR7-deficient mice. Cryosections of the thymi from adult (6- to 8-week-old) wild-type (+/+) and CCR7−/− (−/−) mice were stained with antibodies to CD4 (green) and CD8 (red) to identify DP and SP cells. DP cells appear in yellow. Nuclear staining was performed with 4,6-diamidino-2-phenylindole (DAPI; white). Cortex and medulla topography is reflected by the DAPI stain (left panels). CD4 SP cells are normally found in the medulla of CCR7-deficent animals. (A) A representative region (objective magnification, 4×; numeric aperture [NA], 0.16) is shown of the thymus of CCR7-deficient animals containing several medullary areas, all of them filled with CD4 SP cells (green). Note that even the medullary areas that are misplaced at the outer rim of the organ contain CD4 SP cells (arrows). (B) High magnification picture (objective magnification, 20×/0.75 NA) showing a representative medullary area of the thymus of wild-type (+/+) and CCR7-deficient mice (−/−). Whereas CD4 SP cells are normally found in the medulla of both mouse strains, CD8 SP cells are less abundant in CCR7−/− mice. Images were acquired using an Olympus BX61 microscope. See “Immunohistology” for more image acquisition information. (C) Mean (± SD) of the numbers of CD4 and CD8 SP cells per medullary area. Quantitative data were obtained from analysis of 3 mice from each genotype.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-01-070284/3/m_zh80010810640004.jpeg?Expires=1765894913&Signature=BvFaOpvCJMgPJXy27YvK3wbHGZQkgKwLWenL~REXaF-Bc--2mqucmsyrV5-oJHXN5WSM~3UIGUIJbq4eeg~wVZ0JA9xdHAqdUpzFa7NDmWB8TyLqqLk7G5P9zGukz1i2D~Awd2fr7v1WpCLPia4BDti3mDf9uL2Ru7BU-qFJLuK1grM6zCkDTiSWHroisE3o-x2zFq1W8-lcYqjPdPwHkgmOoV8XFI4sP99SyD-SHA6X2j5JAdB-HbT9Q4HBba5n8zpM92-fDqjvGHfIekSaGjpCzlw-zU8rneg6z9FRNx2kiPqzAua~6Fa4uNGkqDYj3YSZD0QuDfFJ-3SaU7mWkQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal