The process of dendritic cell (DC) maturation, critical for effective DC-based immunotherapy, also alters the proteasome such that peptides presented in the context of HLA class I are generated not by the constitutive proteasome, but by the immunoproteasome. Cytotoxic T lymphocytes (CTLs) induced by such DCs might not optimally recognize tumor cells normally expressing the constitutive proteasome. Using small interfering RNA (siRNA) transfection of DCs to inhibit expression of the 3 inducible immunoproteasome subunits in mature DCs, we found that such DCs expressed increased intracellular levels of constitutive proteasomes and presented an altered repertoire of tumor-antigenic peptides. When DCs generated from the monocytes of 3 patients with melanoma were transfected with immunoproteasome siRNA, induced to mature, and then trans-fected with RNA encoding defined melanoma antigens, these DCs were superior inducers of antigen-specific CTLs against autologous melanoma cells. This alteration of DC proteasome composition, which enhances the ability of mature antigen-loaded DCs to stimulate anti-tumor immune responses, may lead to more effective DC-based tumor immunotherapy.
Introduction
Dendritic cell (DC)–based strategies are increasingly used for cancer immunotherapy. When DCs are loaded with tumor antigens in the form of tumor cell lysates or tumor antigen–encoding DNA or RNA,1,2 antigen processing to generate immunogenic peptides is critical for the induction of antitumor cytotoxic T lymphocyte (CTL) responses. It is the proteasome, the multisubunit complex responsible for degrading ubiquitinylated proteins, that generates these peptides that are presented in the context of HLA class I.
The maturation state of DCs is also critical for stimulating antitumor CTLs and for inducing immune responses against pathogens.3 Immature DCs (iDCs), in contrast, can induce immunologic tolerance, a state undesirable in a host responding to infection or tumor immunotherapy.4
DC maturation and peptide epitope generation by the proteasome are not isolated processes, but are closely linked, as DC maturation also alters the constituents of the proteasome. Specifically, constitutive β subunits X (β5), Y (β1), and Z (β2) are replaced by the inducible β subunits LMP7 (β5i), LMP2 (β1i), and MECL-1 (β2i), respectively.5 The resultant “immunoproteasome” (iP) produces a different repertoire of peptides that is presented by class I molecules on the surface of the mature DC (mDC), compared with the peptide repertoire produced by the constitutive proteasomes (cPs) of iDCs and normal cells that have not been exposed to inflammatory mediators.6
The induction of the iP may be one mechanism to control development of autoimmunity.7 At the site of an infection, for example, inflammatory mediators such as IFN-γ released by cells of the innate immune system8 will induce the iP in nearby cells, altering the repertoire of presented self-peptides and enhancing presentation of pathogen-derived peptides. DCs that have taken up foreign antigens at the site of infection and have been induced to mature by the same inflammatory mediators will migrate to regional lymph nodes and present a similar repertoire of peptides, inducing CTLs specific for peptides generated by the iP. These CTLs will then migrate to the site of infection, killing infected cells exposed to inflammatory mediators, but not recognizing noninfected normal cells, whose repertoire of presented peptides will have been generated by the cP, not the iP, thus limiting both damage to normal tissues and induction of autoimmunity.
Since many of the antigens overexpressed by tumors are self-antigens, this mechanism to limit autoimmunity may limit effectiveness of DC-based cancer immunotherapy. When DCs are loaded with tumor antigens and induced to mature for use as a vaccine, the cP of the DC converts exclusively to the iP.9 These mDCs will potentially stimulate a CTL response against the repertoire of peptides produced by the iP, not the cP. These CTLs would not optimally recognize tumor cells that, due to an absence of IFN-γ exposure,10 or through frequent mutations that prevent iP induction,11 generate peptides for HLA class I presentation using the cP.
In this study, we inhibited the expression of the iP in mDCs using small interfering RNA (siRNA) transfection targeting the 3 inducible iP subunits. DCs with altered iP expression were then evaluated for changes in presentation of defined antigenic peptides. Finally, in an autologous in vitro human melanoma immunotherapy model, the ability of mDCs with altered proteasome expression to stimulate antimelanoma CTLs was evaluated.
Materials and methods
Cells and cell lines
The T-cell clone 381/84, specific for HLA-B51–restricted peptide VPYGSFKHV of the RU1 cellular protein, and the HLA-B51 + BB64-RCC cell line were generously provided by Dr B. J. Van den Eynde (Brussels, Belgium).12 DCs were generated using peripheral blood mononuclear cells (PBMCs) from random American Red Cross blood donors.
Melanoma cell lines were established from patient specimens collected as part of a protocol approved by the Duke University Medical Center institutional review board, and maintained in Iscove modified Dulbecco medium (IMDM) plus 10% fetal bovine serum (FBS). Informed consent was obtained in accordance with the Declaration of Helsinki. These same patients underwent leukapheresis, and PBMCs were cryopreserved. HLA-A2 expression was assessed using A2-specific mAbs and fluorescence-activated cell sorting (FACS). Expression of MART, tyrosinase, gp100, and MAGE-3 was assessed using reverse transcription–polymerase chain reaction (RT-PCR) and tumor-associated antigen (TAA)–specific primers.
iP subunit–specific siRNAs
Small interfering RNA SMARTpools specific for iP subunits LMP2, LMP7, MECL-1, and control siRNA (CsiRNA) were purchased from Dharmacon (Lafayette, CO).
RNA synthesis and isolation
The full-length genes for melanoma TAAs MART, tyrosinase, gp100, MAGE-3, and MAGE-C2 were amplified using RT-PCR and inserted into a plasmid containing a T7 promoter and a polyadenosine “tail,” as previously described.13 Plasmid DNA was used as a template for in vitro RNA transcription using a commercial kit (T7 mMessage machine; Ambion, Austin, TX).
Human monocyte-derived DCs
DCs were generated from adherent monocytes, or from CD14+ monocytes isolated using ferrous conjugated anti-CD14 mAb (Miltenyi Biotec, Auburn, CA).13
Transfection of DCs with siRNA
Immature DCs were transfected with siRNA using electroporation, as previously described for RNA transfection,13,14 with siRNA added to DCs at a total concentration of 1500 nM. Electroporated DCs were transferred to tissue-culture wells with fresh X-vivo 15 media supplemented with IL-4 and granulocyte-macrophage colony-stimulating factor (GM-CSF). At 4 hours following siRNA transfection, DCs were induced to mature by the addition of either LPS (1 μg/mL) or a cytokine cocktail (CC; 10 ng/mL TNF-α, 150 ng/mL IL-6, 10 ng/mL IL-1β, and 1 μg/mL PGE2) to the medium.
Proteasome composition and function of siRNA-transfected and matured DCs were analyzed 24 to 48 hours following transfection. For CTL stimulation, 16 to 20 hours after siRNA transfection and maturation induction, DCs were transfected with RNA encoding melanoma TAAs MART, gp100, tyrosinase, MAGE-3, or MAGE-C2 (or EGFP as a control), using lipofectamine (Invitrogen, Carlsbad, CA).15 After another 24 hours in culture in DC media containing CC, these DCs were used to stimulate CTLs.
Evaluation of iP subunit gene expression levels
Total RNA isolated from siRNA-transfected DCs using the RNeasy kit (Qiagen, Valencia, CA) was used as a template for cDNA synthesis using Superscript II (Invitrogen) and oligo-dT. Quantitative PCR was performed using SYBR green (Bio-Rad, Hercules, CA) and analyzed using an IQ Cycler (Bio-Rad) and primer pairs specific for LMP2, LMP7, MECL-1, X, Y, Z, and cyclophilin B (Invitrogen).
Detection of intracellular proteasome subunits
DC lystates were subjected to 10% PAGE and Western transfer. Membranes were then incubated with primary mAbs specific for LMP7, LMP2, X, and Y (BioMol, Plymouth Meeting PA), with detection by chemiluminescence.
For intracellular FACS, DCs derived from CD14+ monocytes were fixed in 2% paraformaldehyde, and after microwave treatment were permeabilized using 0.1% saponin and incubated with primary mAbs SY-5, SJJ-3, SY-1, and HB2 with specificity for proteasome subunits Y, X, LMP2, and LMP7, respectively (all generously provided by S. Ferrone, Roswell Park Cancer Institute, Buffalo, NY).16,17 DCs were then incubated with APC-conjugated goat F(ab′)2 anti-mouse IgG and analyzed by flow cytometry. Similarly prepared DCs were also evaluated for LMP2 expression by fluorescence microscopy.
Effector T-cell clone epitope recognition assay
B51+ iDCs were transfected with siRNAs targeting LMP2, LMP7, and MECL-1 (iPsiRNA) or with CsiRNA, and maturation was induced using CC. At 24 to 48 hours later, these DCs, or positive control BB64-RCC cells, were incubated with clone 381/84 T cells in culture for 16 hours. Levels of TNF-α in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Induction of CTLs against the RU1 peptide
HLA-B51+ DCs were transfected with either CsiRNA or iPsiRNA and left untreated as iDCs or induced to mature using CC. These DCs were then incubated with the 19-amino acid (aa) RU1 polypeptide TGSTAVPYGSFKHVDTRLQ corresponding to amino acids 29 to 47 of the RU1 renal cell carcinoma antigen containing the HLA-B51–restricted peptide epitope VPYGSFKHV generated from RU1 exclusively through processing by the cP and not the iP.12 After 4 hours, these DCs were washed and added to autologous T cells in culture. After a single restimulation, these T-cell cultures were evaluated by IFN-γ enzyme-linked immunospot (ELISPOT) and Europium release assays.
Induction of CTLs against melanoma tumor–associated antigens
Immature DCs were transfected with either CsiRNA or iPsiRNA. After the induction of maturation using CC, these DCs were then transfected with RNA-encoding melanoma TAAs MART, tyrosinase, gp100, MAGE-3, or MAGE-C2, then used to stimulate autologous T cells in vitro.
Briefly, T cells isolated from autologous nonadherent lymphocytes were resuspended in T-cell media consisting of RPMI supplemented with 25 mM HEPES, 10% FBS, penicillin/streptomycin, glutamine, sodium pyruvate, nonessential amino acids, and β-mercaptoethanol at a concentration of 6.6 × 106 cells/mL. A total of 3 mL of these lymphocytes (20 × 106) were added to 3 mL of RNA-transfected DCs (2 × 106), and 2-mL aliquots of the mixed cells were plated in triplicate in wells of a 12-well tissue-culture plate, yielding a final lymphocyte-DC ratio of 10:1. IL-2 at 20 U/mL was added beginning on day 7 and then twice per week thereafter. Each T-cell culture was restimulated with RNA-transfected DCs at 7- to 10-day intervals. At 7 to 10 days following restimulation, the in vitro–stimulated T cells were harvested and evaluated for CTL effector function using IFN-γ ELISPOT and/or Europium release assays.
IFN-γ ELISPOT
IFN-γ release ELISPOT assays were performed using a kit (BD Biosciences, Palo Alto, CA), in triplicate, using RNA-transfected autologous DCs or cell-line targets and a 16- to 18-hour incubation. To assess peptide-specific responses, HLA-A2–resticted peptides alone were added to effector T cells. Peptides included EAAGIGILTV, ITDQVPFSV, and YMDGTMSQV (corresponding to MART-126-35, gp100209-217, and tyrosinase369-377, respectively), known to be generated by the cP and not the iP; tyrosinase1-9 (mllavlycl), generated independently of proteasome identity; and MAGE-C2336-344 (ALKDVEERV), generated by the iP but destroyed by the cP.18 Plates were processed and developed according to the manufacturer's protocol, then scanned and counted using an ImmunoSpot Series Analyzer (Cellular Technology, Cleveland, OH).
Europium release assay
Lytic activity of effector T cells was determined using a Europium release assay.19 Tumor cell lines and autologous DCs transfected with RNA served as targets.
Statistical analysis
All assays were preformed in duplicate or triplicate, and values are plotted as means plus or minus SD unless otherwise indicated. Values were compared using an unpaired t test (GraphPad Software, San Diego, CA).
Results
Alteration of DC intracellular proteasome subunit composition
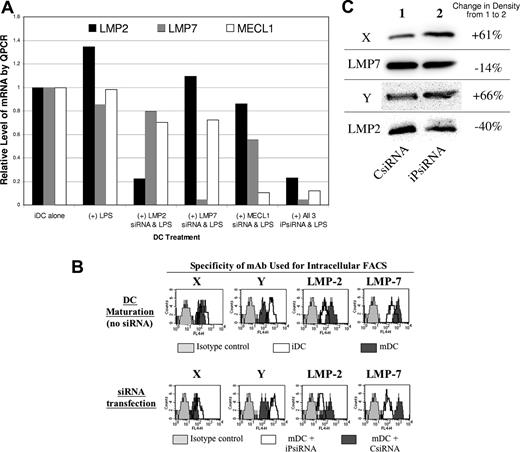
Human iDCs were transfected with either CsiRNA or a mixture of siRNAs targeting each inducible iP subunits. At 4 hours later, DC maturation was induced using LPS, and after 24 hours, mRNA levels of the iP subunits were assessed by quantitative PCR (QPCR). As shown in Figure 1A, siRNA transfection specifically down-regulated expression of mRNA encoding each of the inducible iP subunits. Transfection of DCs with a combination of siRNA targeting all 3 inducible iP subunits (iPsiRNA) resulted in down-regulation of each subunit to a similar extent as was observed when each subunit was targeted individually. For this reason, a combination of siRNAs targeting all 3 of inducible iP subunits was used for transfection in all subsequent experiments.
iPsiRNA-mediated down-modulation of inducible iP subunits in DCs. (A) iDCs were electroporated with the indicated siRNAs (500 nM of each individual iP subunit–specific siRNA, with control siRNA added as needed to a final total concentration of 1500 nM). At 4 hours later, LPS was added to the siRNA-transfected DCs. After 24 hours of maturation induction, DC RNA was extracted and quantitative PCR was performed to determine relative levels of mRNA encoding iP subunits LMP2, LMP7, and MECL-1. The data presented are representative of 3 separate experiments, each with similar results. (B) Using DCs derived from CD14+ monocytes, intracellular expression of cP subunits X and Y and iP subunits LMP2 and LMP7 was assessed using specific mAbs, as well as isotype-matched control mAbs, and an APC-conjugated secondary antibody, detected as FL4. In the top panel, iDCs and mDCs (induced to mature using a CC for 24 hours in culture) were analyzed, while in the bottom panel, the DCs analyzed were transfected with either CsiRNA or iPsiRNA and induced to mature for 24 hours using the CC. The data presented are representative of 2 experiments, each with similar results. (C) Specific subunit composition of 20S proteasomes from mDCs transfected with either CsiRNA or iPsiRNA was assessed by Western blotting of cell lysates. Densitometry measurements were compared as indicted. This experiment was performed 3 times, with similar results.
iPsiRNA-mediated down-modulation of inducible iP subunits in DCs. (A) iDCs were electroporated with the indicated siRNAs (500 nM of each individual iP subunit–specific siRNA, with control siRNA added as needed to a final total concentration of 1500 nM). At 4 hours later, LPS was added to the siRNA-transfected DCs. After 24 hours of maturation induction, DC RNA was extracted and quantitative PCR was performed to determine relative levels of mRNA encoding iP subunits LMP2, LMP7, and MECL-1. The data presented are representative of 3 separate experiments, each with similar results. (B) Using DCs derived from CD14+ monocytes, intracellular expression of cP subunits X and Y and iP subunits LMP2 and LMP7 was assessed using specific mAbs, as well as isotype-matched control mAbs, and an APC-conjugated secondary antibody, detected as FL4. In the top panel, iDCs and mDCs (induced to mature using a CC for 24 hours in culture) were analyzed, while in the bottom panel, the DCs analyzed were transfected with either CsiRNA or iPsiRNA and induced to mature for 24 hours using the CC. The data presented are representative of 2 experiments, each with similar results. (C) Specific subunit composition of 20S proteasomes from mDCs transfected with either CsiRNA or iPsiRNA was assessed by Western blotting of cell lysates. Densitometry measurements were compared as indicted. This experiment was performed 3 times, with similar results.
Using intracellular FACS, we first confirmed that with the induction of DC maturation, intracellular expression of inducible iP subunits LMP2 and LMP7 was increased, while the expression of the corresponding cP subunits Y and X was reduced (Figure 1B top panel). In comparison with CsiRNA transfection, when mature monocyte-derived DCs had been transfected with iPsiRNA, intracellular expression of inducible iP subunits LMP2 and LMP7 was decreased, with concurrent increased intracellular expression of cP subunits Y and X (Figure 1B bottom panel). Fluorescence microscopic evaluation of similarly treated DCs demonstrated that the perinuclear accumulation of iP, seen in mDCs that had been either untransfected or transfected with CsiRNA, was no longer visualized after transfection with iPsiRNA (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As shown in Figure 1C, Western blot analysis of DC lysates also confirmed reduced levels of iP subunits LMP2 and LMP7, with concomitant increased levels of cP subunits X and Y in mDCs after iPsiRNA transfection.
In addition, DCs transfected with either CsiRNA or iPsiRNA were metabolically labeled during maturation, and intact 20S proteasomes were immunoprecipitated using a mAb specific for the α2 subunit, present in both cP and iP, then subjected to 2D gel electrophoresis. As shown in Figure S2, after iPsiRNA transfection (bottom gel), expression of all 3 of the cP subunits (X, Y, and Z) normally replaced by the inducible iP subunits after the induction of DC maturation was increased when compared with DCs transfected with CsiRNA (top gel).
To exclude the possibility that siRNA transfection interfered with the induction of maturation, DC phenotypic analysis was performed using FACS analysis. The induction of DC maturation using the CC resulted in surface expression of both CD25 and CD83, as well as increased surface levels of CD86 and HLA class II (Figure S3). Importantly, the mature phenotype of DCs cultured in the presence of the CC was not altered by transfection with either CsiRNA or iPsiRNA. Stimulatory capacity of mDCs in allogeneic mixed lymphocyte reaction (MLR) assays as well as DC migration in response to the chemokine CCL21 were both unaltered by iPsiRNA transfection (data not shown).
iP down-modulation alters DC peptide presentation
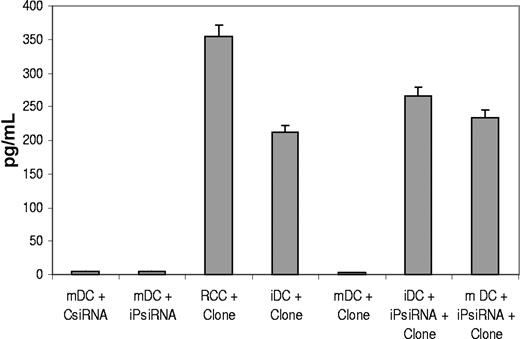
The ability of iPsiRNA transfection to alter DC antigen processing and peptide presentation was evaluated using an HLA-B51–restricted CTL clone specific for a peptide derived from the ubiquitous RU1 self-protein and generated exclusively by cP processing and not by the iP.12 As shown in Figure 2, this clone secreted TNF-α in response to both the B51+ autologous renal cell cancer (RCC) cell line and B51+ iDCs, both of which express the cP and therefore can generate and present the RU1-derived peptide. In contrast, the clone did not secrete TNF-α in response to mDCs, which express the iP. Immature HLA-B51+ DCs that were transfected with siRNAs targeting the iP induced higher levels of TNF-α secretion when compared with iDCs alone. Most importantly, after iPsiRNA transfection, mature DCs, previously unable to induce TNF-α secretion by the clone, now stimulated TNF-α secretion at levels comparable with that seen with iDCs.
iPsiRNA transfection alters DC peptide antigen presentation. TNF-α secretion by a CTL clone specific for an RU1-derived peptide epitope generated exclusively by the cP and not the iP and presented in the context of HLA-B51 was assessed. B51+ DCs were used as targets as either iDCs or mDCs. Maturation was induced using a CC (TNF-α, IL-6, IL-1β, and PGE2) for 48 hours after electroporation of the DCs with siRNAs targeting the iP subunits (iPsiRNA) or control siRNA (CsiRNA). Tissue-culture supernatants were harvested 16 hours after the addition of the CTL clone (+Clone) and TNF-α concentration was determined by ELISA and expressed as mean OD450 (± SEM). The results are representative of 3 independent experiments, each with similar results.
iPsiRNA transfection alters DC peptide antigen presentation. TNF-α secretion by a CTL clone specific for an RU1-derived peptide epitope generated exclusively by the cP and not the iP and presented in the context of HLA-B51 was assessed. B51+ DCs were used as targets as either iDCs or mDCs. Maturation was induced using a CC (TNF-α, IL-6, IL-1β, and PGE2) for 48 hours after electroporation of the DCs with siRNAs targeting the iP subunits (iPsiRNA) or control siRNA (CsiRNA). Tissue-culture supernatants were harvested 16 hours after the addition of the CTL clone (+Clone) and TNF-α concentration was determined by ELISA and expressed as mean OD450 (± SEM). The results are representative of 3 independent experiments, each with similar results.
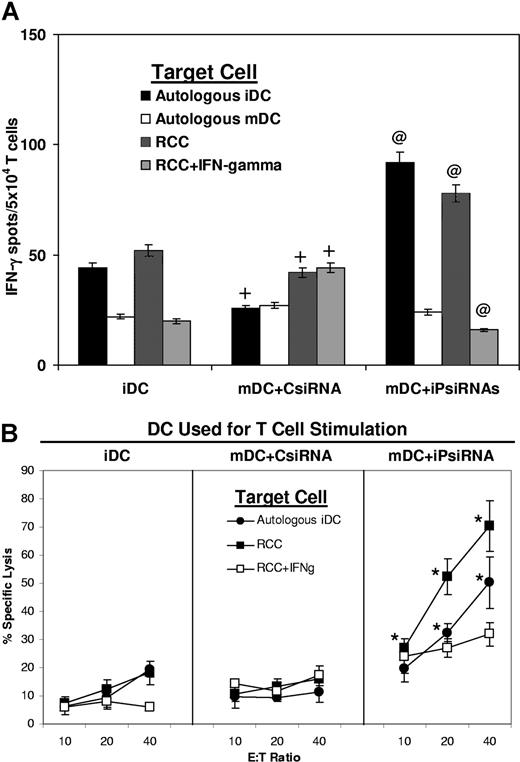
We next evaluated whether iPsiRNA transfection would alter the ability of HLA-B51+ DCs to stimulate a CTL response. For these experiments, immature HLA-B51+ DCs were left untreated (iDCs) or were transfected with either CsiRNA or iPsiRNA and induced to mature. All 3 of these DC preparations were then loaded with a 19-aa RU1 polypeptide containing the immunogenic B51-restricted 10-aa RU1 peptide generated exclusively by the cP and not the iP. As assessed by IFN-γ ELIPSPOT (Figure 3A), a significantly higher number of CTLs secreting IFN-γ in response to both autologous iDCs and the HLA-B51+ RCC cell line was observed when stimulator DCs had been transfected with iPsiRNA. As expected, these CTLs did not show an increased response against mature DCs (that express the iP) or against RCC cells that had been incubated with IFN-γ to induce iP expression. Lytic activity against the HLA-B51+ RCC cell line was also significantly increased when T cells had been stimulated using mDCs transfected with iPsiRNA rather than control siRNA (Figure 3B). When RCC cells had been previously incubated with IFN-γ to induce iP expression, these target cells were less susceptible to lysis.
iPsiRNA-transfected DCs simulate the induction of CTLs specific for a defined antigen. CTLs were induced by stimulation of autologous T cells with B51+ DCs loaded with the 19-aa precursor RU-1 polypeptide after no treatment (iDC), after transfection with control siRNA and the induction of DC maturation using CC (mDC + CsiRNA), or after transfection with siRNA targeting the 3 inducible iP subunits followed by induction of maturation (mDC + iPsiRNA). After 1 restimulation, T-cell IFN-γ release in response to the indicated target cells was assessed by ELISPOT (A), and cytolytic activity was assessed using the Europium release assay and either autologous iDCs (autologous iDC), the B51+ renal cell carcinoma cell line (RCC), or RCC preincubated for 1 week with IFN-γ to induce iP expression (RCC + IFN-γ) as targets (B). Values are plotted as means (± SD); *P < .05; @P < .01 for the comparison between T-cell activity induced by mDCs + iPsiRNA versus mDCs + CsiRNA; and +P < .01 for the comparison between T cells induced by mDCs + CsiRNA versus iDCs.
iPsiRNA-transfected DCs simulate the induction of CTLs specific for a defined antigen. CTLs were induced by stimulation of autologous T cells with B51+ DCs loaded with the 19-aa precursor RU-1 polypeptide after no treatment (iDC), after transfection with control siRNA and the induction of DC maturation using CC (mDC + CsiRNA), or after transfection with siRNA targeting the 3 inducible iP subunits followed by induction of maturation (mDC + iPsiRNA). After 1 restimulation, T-cell IFN-γ release in response to the indicated target cells was assessed by ELISPOT (A), and cytolytic activity was assessed using the Europium release assay and either autologous iDCs (autologous iDC), the B51+ renal cell carcinoma cell line (RCC), or RCC preincubated for 1 week with IFN-γ to induce iP expression (RCC + IFN-γ) as targets (B). Values are plotted as means (± SD); *P < .05; @P < .01 for the comparison between T-cell activity induced by mDCs + iPsiRNA versus mDCs + CsiRNA; and +P < .01 for the comparison between T cells induced by mDCs + CsiRNA versus iDCs.
Induction of TAA-specific immune responses by DCs is enhanced by iP down-modulation
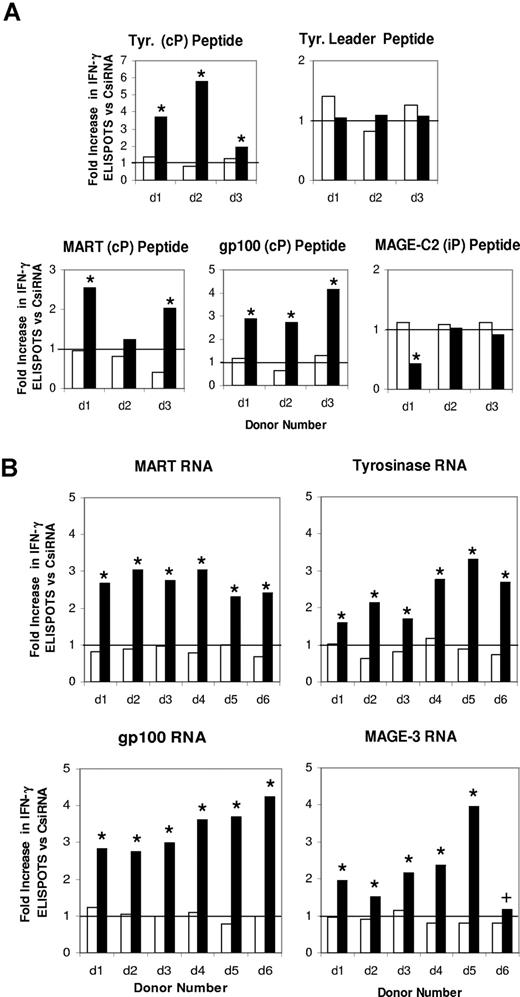
To determine if iP down-modulation altered the ability of TAA RNA–transfected DCs to stimulate TAA-specific immunity, iDCs were generated from 3 HLA-A2+ donors. After transfection with CsiRNA or iPsiRNA and the induction of maturation, these DCs were transfected with RNA encoding the TAAs MART, tyrosinase, gp100, or MAGE-C2, then used to stimulate autologous T cells in vitro. After 2 restimulations with identically prepared DCs, INF-γ ELISPOT assays were performed in response to defined HLA-A2–restricted peptides. As shown in Figure 4A, significantly higher numbers of IFN-γ–secreting CTLs specific for the cP-generated MART peptide were seen in 2 of the 3 donors when stimulator DCs were transfected with iPsiRNA. For the TAAs gp100 and tyrosinase, responses specific for the cP-generated gp100 and tyrosinase peptides in all 3 donors were significantly increased when stimulator DCs were transfected with iPsiRNA. The frequencies of CTLs specific for the tyrosinase leader–derived peptide1,,,,,,,–9 that is not differentially generated by either the cP or the iP were equivalent in all 3 donors whether stimulator DCs had been transfected with either CsiRNA or iPsiRNA. The frequencies of CTLs specific for the MAGE-C2 peptide generated exclusively by the iP were unchanged in 2 of 3 donors, and significantly reduced in 1 donor when stimulatory DCs were transfected with iPsiRNA. In all experiments, nonspecific IFN-γ background reactivity was unchanged when TAA RNA-transfected DC stimulators were cotransfected with iPsiRNA.
Enhanced induction of IFN-γ–secreting TAA-specific T cells when stimulator DCs are transfected with iPsiRNA. (A) DCs generated from 3 HLA-A2+ donors were transfected with either CsiRNA or iPsiRNA. After the induction of maturation with CC, these DCs were transfected with RNA encoding the indicated TAA and used to stimulate autologous T cells in vitro. After 2 restimulations with identically prepared DCs, IFN-γ ELISPOT assays were performed using the indicated defined HLA-A2–restricted peptides generated by the cP, the iP, or independently of the identity of the proteasome (Tyr. Leader). ■ indicates the fold increase in the number of peptide-specific ELISPOTs induced by iPsiRNA-transfected DCs versus CsiRNA-transfected DCs; □, the fold increase in nonspecific background ELISPOTS against control peptide for T cells induced by iPsiRNA-transfected versus CsiRNA-transfected DCs. (B) DCs generated from 6 individual HLA-A2− donors were transfected with either CsiRNA or iPsiRNA. After the induction of maturation, these DCs were transfected with RNA encoding the indicated TAA and used to stimulate autologous T cells in vitro. After a single restimulation with identically prepared DCs, IFN-γ ELISPOT assays were performed using autologous DCs transfected with the specific TAA RNA or a negative control RNA as targets. ■ indicates the fold increase in the number of TAA-specific ELISPOTS induced by iPsiRNA-transfected DCs versus CsiRNA-transfected DCs; □, the fold increase in nonspecific background ELISPOTS against control RNA–transfected autologous DC targets for T cells induced by iPsiRNA versus CsiRNA-transfected DCs. Absolute numbers of IFN-γ ELISPOTS for each depicted ratio for each individual donor were compared by t test; *P < .01; +P < .05. Horizontal bars indicate unity.
Enhanced induction of IFN-γ–secreting TAA-specific T cells when stimulator DCs are transfected with iPsiRNA. (A) DCs generated from 3 HLA-A2+ donors were transfected with either CsiRNA or iPsiRNA. After the induction of maturation with CC, these DCs were transfected with RNA encoding the indicated TAA and used to stimulate autologous T cells in vitro. After 2 restimulations with identically prepared DCs, IFN-γ ELISPOT assays were performed using the indicated defined HLA-A2–restricted peptides generated by the cP, the iP, or independently of the identity of the proteasome (Tyr. Leader). ■ indicates the fold increase in the number of peptide-specific ELISPOTs induced by iPsiRNA-transfected DCs versus CsiRNA-transfected DCs; □, the fold increase in nonspecific background ELISPOTS against control peptide for T cells induced by iPsiRNA-transfected versus CsiRNA-transfected DCs. (B) DCs generated from 6 individual HLA-A2− donors were transfected with either CsiRNA or iPsiRNA. After the induction of maturation, these DCs were transfected with RNA encoding the indicated TAA and used to stimulate autologous T cells in vitro. After a single restimulation with identically prepared DCs, IFN-γ ELISPOT assays were performed using autologous DCs transfected with the specific TAA RNA or a negative control RNA as targets. ■ indicates the fold increase in the number of TAA-specific ELISPOTS induced by iPsiRNA-transfected DCs versus CsiRNA-transfected DCs; □, the fold increase in nonspecific background ELISPOTS against control RNA–transfected autologous DC targets for T cells induced by iPsiRNA versus CsiRNA-transfected DCs. Absolute numbers of IFN-γ ELISPOTS for each depicted ratio for each individual donor were compared by t test; *P < .01; +P < .05. Horizontal bars indicate unity.
iDCs were then generated from the PBMCs of 6 HLA-A2− donors, transfected with either CsiRNA or iPsiRNA, induced to mature, then transfected with RNA encoding the melanoma TAAs MART, tyrosinase, gp100, and MAGE-3. These DCs were used to stimulate autologous T cells in vitro. After a single restimulation with identically prepared DCs, T cells were evaluated by IFN-γ ELISPOT against autologous DCs transfected with TAA-encoding RNA. As shown in Figure 4B, for all 6 donors, iPsiRNA-transfected DCs induced significantly higher numbers of TAA-specific IFN-γ–secreting T cells compared with control siRNA-transfected DCs for each TAA. The nonspecific background number of IFN-γ ELISPOT was not significantly increased in any experiment by DC transfection with iPsiRNA versus CsiRNA.
Induction of antimelanoma immune responses in an in vitro immunotherapy model
We next evaluated the ability of iPsiRNA-transfected DCs to stimulate enhanced antimelanoma CTL activity in an in vitro autologous melanoma immunotherapy model. A total of 3 melanoma cell lines previously generated from clinical patient samples of metastatic melanoma were characterized for expression of melanoma TAAs MART, tyrosinase, gp100, and MAGE-3.
Cryopreserved PBMCs from each patient, from whom these 3 melanoma lines were derived, were used to generate autologous DCs in vitro. After transfection with either CsiRNA or iPsiRNA, and the induction of maturation, these DCs were then transfected with RNA encoding each of the melanoma TAAs, depending upon the expression by the autologous melanoma cell line. These DCs were then used to stimulate autologous CTLs in culture.
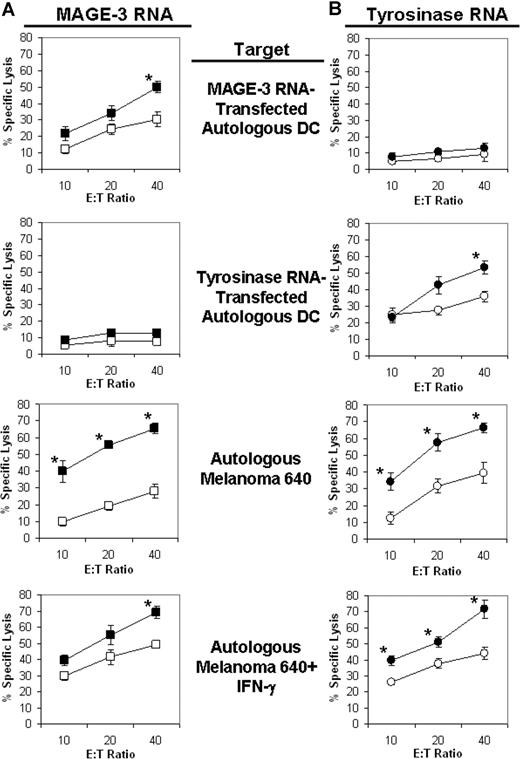
The cell line derived from patient 640 was found to express TAAs MAGE-3 and tyrosinase. Autologous mature siRNA-transfected DCs from this patient were therefore transfected with RNA encoding either MAGE-3 or tyrosinase, and used to stimulate autologous T cells. After a single restimulation, the resultant CTL cultures were evaluated for cytotoxicity against a variety of targets. As shown in Figure 5, when assessed with MAGE-3 and tyrosinase RNA–transfected DC targets, the highest antigen-specific lytic activity was induced when MAGE-3 (Figure 5A) and tyrosinase (Figure 5B) RNA–transfected DC stimulators had been cotransfected with iPsiRNA (versus CsiRNA).
Lytic activity of CTLs generated from patient 640. Autologous iDCs were generated from cryopreserved PBMCs and then transfected with either CsiRNA (□/○) or iPsiRNA (■/●). After the induction of maturation, these DCs were transfected with either MAGE-3 RNA (A) or tyrosinase RNA (B) and then used to stimulated autologous T cells in culture. After 1 restimulation, lytic activity was determined in duplicate against autologous DCs transfected with either MAGE-3 RNA or tyrosinase RNA, or the autologous melanoma cell line 640 alone or treated with IFN-γ for 3 days. Results are plotted as mean values (± SD); *P < .05 for the comparison between lytic activity of T cells induced by CsiRNA-transfected stimulator DCs versus iPsiRNA-transfected stimulator DCs measured against the same target.
Lytic activity of CTLs generated from patient 640. Autologous iDCs were generated from cryopreserved PBMCs and then transfected with either CsiRNA (□/○) or iPsiRNA (■/●). After the induction of maturation, these DCs were transfected with either MAGE-3 RNA (A) or tyrosinase RNA (B) and then used to stimulated autologous T cells in culture. After 1 restimulation, lytic activity was determined in duplicate against autologous DCs transfected with either MAGE-3 RNA or tyrosinase RNA, or the autologous melanoma cell line 640 alone or treated with IFN-γ for 3 days. Results are plotted as mean values (± SD); *P < .05 for the comparison between lytic activity of T cells induced by CsiRNA-transfected stimulator DCs versus iPsiRNA-transfected stimulator DCs measured against the same target.
Most importantly, the cytolytic activity against the autologous melanoma cell line was markedly greater when stimulator DCs had been transfected with iPsiRNA rather than control siRNA and then transfected with either MAGE-3 (Figure 5A) or tyrosinase (Figure 5B) RNA. Interestingly, preincubation of the melanoma cell line with IFN-γ to induce expression of the iP did not markedly reduce the ablility of these melanoma cell targets to be lysed by CTLs induced by DCs transfected with iPsiRNA.
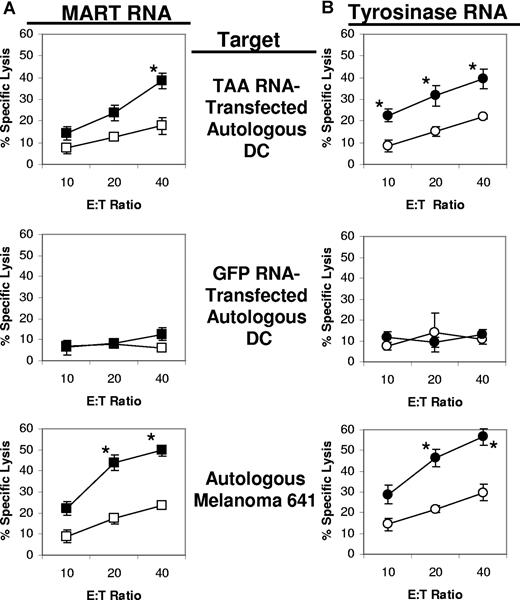
For the 641 cell line (MART+ and tyrosinase+), CTLs were induced using the same procedure, but RNA encoding either MART or tyrosinase was used for transfection. As shown in Figure 6A, when MART RNA–transfected DCs were used to stimulate autologous T cells, the highest lytic activity against MART RNA–transfected DCs was induced with the DCs that had been cotransfected with iPsiRNA. These same CTL cultures also exhibited the highest lytic activity against the autologous 641 melanoma cell line. A similar pattern of CTL induction and specific lytic activity was noted for the CTLs induced by tyrosinase RNA–transfected DCs cotransfected with iPsiRNA versus CsiRNA (Figure 6B).
Lytic activity of CTLs generated from patient 641. Autologous iDCs were transfected with either CsiRNA (□/○) or iPsiRNA (■/●). After the induction of DC maturation, these DCs were transfected with either MART RNA (A) or tyrosinase RNA (B) and then used to stimulate autologous T cells in culture. After 1 restimulation, lytic activity was determined in duplicate against autologous DCs transfected with TAA RNA (Mart RNA in panel A and tyrosinase RNA in panel B) or GFP (control) RNA, and the autologous melanoma cell line 641 derived from this patient. Results are plotted as mean values (± SD); *P < .05.
Lytic activity of CTLs generated from patient 641. Autologous iDCs were transfected with either CsiRNA (□/○) or iPsiRNA (■/●). After the induction of DC maturation, these DCs were transfected with either MART RNA (A) or tyrosinase RNA (B) and then used to stimulate autologous T cells in culture. After 1 restimulation, lytic activity was determined in duplicate against autologous DCs transfected with TAA RNA (Mart RNA in panel A and tyrosinase RNA in panel B) or GFP (control) RNA, and the autologous melanoma cell line 641 derived from this patient. Results are plotted as mean values (± SD); *P < .05.
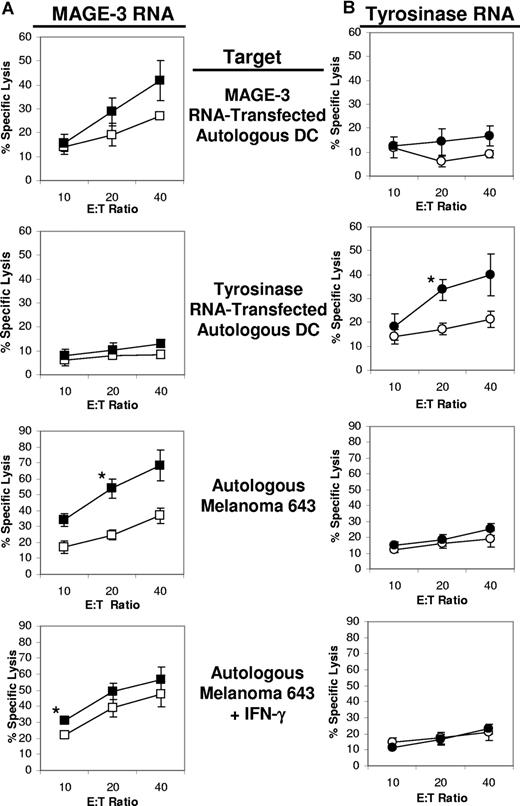
The cell line derived from patient 643 only expressed the TAA MAGE-3. Autologous DCs were first transfected with either control siRNA or iPsiRNA and then induced to mature. These DCs were then transfected with RNA encoding either MAGE-3 or tyrosinase (as a negative control) and used to induce CTLs. As shown in Figure 7A, for CTLs induced using DCs transfected with MAGE-3 RNA, the highest lytic activity against MAGE-3 RNA–transfected DCs was observed from CTL cultures in which stimulator DCs were cotransfected with iPsiRNA, rather than CsiRNA. For tyrosinase RNA–transfected DC stimulators (Figure 7B), iPsiRNA transfection also led to the induction of the highest specific cytolytic activity against tyrosinase RNA–transfected autologous DC targets. Consistent with the expression of MAGE-3 by line 643, lysis of this autologous melanoma cell line was also highest when CTLs were induced using DCs that were cotransfected with iPsiRNA and MAGE-3 RNA. For CTLs induced against tyrosinase, no lytic activity against melanoma 643 was observed, consistent with the lack of expression of this TAA by these melanoma cells. Interestingly, the induction of CTLs against melanoma 643 targets preincubated with IFN-γ was not diminished when DC stimulators had been transfected with iPsiRNA compared with CsiRNA.
Lytic activity of CTLs generated from patient 643. Autologous iDCs were transfected with either CsiRNA (□/○) or iPsiRNA (■/●). After the induction of DC maturation, these DCs were transfected with either MAGE-3 RNA (A) or tyrosinase RNA (B) and then used to stimulate autologous T cells in culture. After 1 restimulation, lytic activity was determined in duplicate against autologous DCs transfected with RNA encoding either MAGE-3 or tyrosinase, and against the autologous melanoma cell line 643 left untreated or preincubated with IFN-γ for 3 days in culture. Results are plotted as mean values (± SD); *P < .05.
Lytic activity of CTLs generated from patient 643. Autologous iDCs were transfected with either CsiRNA (□/○) or iPsiRNA (■/●). After the induction of DC maturation, these DCs were transfected with either MAGE-3 RNA (A) or tyrosinase RNA (B) and then used to stimulate autologous T cells in culture. After 1 restimulation, lytic activity was determined in duplicate against autologous DCs transfected with RNA encoding either MAGE-3 or tyrosinase, and against the autologous melanoma cell line 643 left untreated or preincubated with IFN-γ for 3 days in culture. Results are plotted as mean values (± SD); *P < .05.
Discussion
In summary, we have found that the iP of the mDC can be down-modulated by transfection with siRNA targeting the inducible iP subunits LMP2, LMP7, and MECL-1. These mature iPsiRNA-transfected DCs show reduced intracellular expression of inducible iP subunits and concomitant increased intracellular expression of cP subunits. These DCs also functionally demonstrate altered antigen processing and antigenic peptide presentation, consistent with replacement of iP with cP. Most important, we demonstrate that DCs transfected with iPsiRNA to down-modulate iP expression, and then induced to mature and cotransfected with RNA encoding defined melanoma TAAs, stimulate enhanced antimelanoma CTL activity.
Peptides generated by proteasome-mediated degradation of intracellular proteins are the major source of peptides presented by HLA class I major histocompatibility complex (MHC) molecules on the cell surface.5 Intracellular proteins are first targeted for destruction by ubiquitinylation, a process that covalently attaches ubiquitin molecules to lysine residues. Ubiquitinylated proteins then enter the 26S proteasome, where they are unfolded by the 19S proteasome cap structure and then degraded into peptides by the 20S proteasome catalytic core.
When both DCs and normal nonlymphoid cells are exposed to IFN-γ, or when DCs are induced to mature, constitutive β proteasome catalytic subunits X (β5), Y (β1), and Z (β2) are replaced by the inducible β catalytic subunits LMP7 (β5i), LMP2 (β1i), and MECL-1 (β2i), respectively, in the 20S proteasome core.9,20 The resultant iP produces a different repertoire of peptides that is presented by class I molecules on the surface of the cell when compared with the peptide repertoire produced by the cP of both iDCs and normal cells that have not been exposed to inflammatory mediators such as IFN-γ.7 Specifically, the iP exhibits increased chymotrypsin-like activity (due to replacement of X by LMP7), variable changes in trypsin-like activity (due to replacement of Z by MECL-1), and reduced caspase-like activity (due to replacement of Y by LMP2) when compared with the activity of the cP. Studies have indicated that the iP is more efficient at generating a variety of immunogenic peptides, but these peptides are almost exclusively derived from proteins expressed by pathogens, not tumors.7
Recently, Chapiro et al have shown that the enhanced chymotrypsin-like activity of the iP, compared with the cP, destroys several tumor-antigenic peptides, including peptides derived from melanoma TAAs tyrosinase and gp100, which can therefore not be presented by mature tumor antigen–loaded DCs.18 Earlier work by Morel et al clearly identified specific tumor-antigenic MHC-restricted peptides, including the EAAGIGILTV immunogenic peptide derived from the melanoma TAA MART that can be recognized by T cells but are not generated in cells that exclusively contain the iP. Instead, these peptides are generated by the cP.12
In our experiments where DCs were derived from HLA-A2+ donors and DC stimulators were cotransfected with RNA encoding TAAs MART, tyrosinase, and gp100 (Figure 4A), we confirmed that normally mature TAA RNA–transfected DCs do not induce marked CTL responses against 3 cP-generated TAA-derived peptides. Thus, if MART, tyrosinase, or gp100 RNA–transfected DCs exclusively expressing the iP were used for immunotherapy, CTLs might not be generated against these potentially immunogenic TAA-derived A2-restricted peptides.
In contrast, iPsiRNA transfection of stimulator DCs induced increased numbers of antigen-specific CTLs against these 3 cP-generated A2-restricted peptides, while not reducing the induction of CTLs specific for a tyrosinase leader–derived peptide generated independently of the identity of the proteasome or (in 2 of 3 donors) for a MAGE-C2–derived peptide generated by the iP and destroyed by the cP. These findings suggest that DCs treated with iPsiRNA express increased levels of cP, but also continue to express some functional iP. It is possible that the reduced response against the MAGE-C2–derived peptide was seen with donor 1 because of less “residual” iP activity in this particular donor. Most important with respect to cancer immunotherapy, our findings suggest that iPsiRNA transfection does not necessarily eliminate the induction of CTLs against iP-derived epitopes. In addition, the significant increase in the induction of immune responses against cells expressing the entire TAA protein (RNA-transfected DCs and melanoma cells) by DCs transfected with iPsiRNA suggest that even if responses against some iP-generated peptides are reduced, the overall antigen-specific immune response is enhanced when the iP of the mDC is reduced by siRNA transfection.
Use of such DCs with altered proteasome composition and function for clinical immunotherapy would therefore potentially induce a CTL response against defined as well as other previously unidentified cP-generated peptides. This in turn would potentially lead to enhanced immune-mediated destruction of tumor cells that, for a variety of reasons, do not normally express the iP.
For example, some tumors may not be exposed to IFN-γ in vivo, as demonstrated by Burke and associates for patient ovarian cancer biopsy specimens.10 While an effective in vivo antitumor immune response with tumor infiltration by natural killer (NK) cells and/or antigen-specific CTLs would result in exposure of the tumor cells to IFN-γ, and the theoretical induction of iP in tumor cells, such antitumor immune responses are not often observed, even after tumor immunotherapy. If such a vigorous antitumor immune response were to occur, further tumor immunotherapy using any approach might be unnecessary. Even in the presence of IFN-γ, it has recently been demonstrated that tumor cells, including melanoma, have developed mechanisms to prevent the induction of the iP,11 perhaps to avoid recognition by CTLs induced against iP-generated peptides.
When evaluating the induction of antitumor immune responses, many investigators overlook the occurrence of proteasome-mediated “mistargeting” by incubating target cells for in vitro assays with IFN-γ. As demonstrated in Figures 5 and 7, CTLs stimulated by DCs transfected with CsiRNA, rather than iPsiRNA, showed higher lytic activity against melanoma targets when these melanoma cells were preincubated with IFN-γ to induce iP expression. By incubating target cells with IFN-γ, the apparent in vitro antitumor CTL activity induced by normal mDCs will be enhanced. However, the actual CTL activity against tumor cells, which would normally not be exposed to IFN-γ in vivo and thus would normally present peptides generated by the cP, not the iP, would be lower. It is important to note that when stimulator DCs were transfected with iPsiRNA in our experiments, the induced CTLs demonstrated enhanced antigen-specific immune responsiveness against target melanoma cells, regardless of whether these target cells had been preincubated with IFN-γ to induce the iP.
When proteasomes isolated from immature monocyte-derived human DCs were evaluated by Macagno et al, both cP and iP were observed.9 Even in iDCs, mRNA and protein expression levels of iP subunits LMP2, LMP7, and MECL-1 are all up-regulated.21 Studies have also demonstrated that upon exposure to IFN-γ, or the induction of maturation using LPS in DCs, enhanced incorporation of iP subunits into proteasomes occurs, with low levels of additional transcriptional induction of LMP7 and MECL-1, and moderate increases in mRNA encoding LMP2 as well as the PA28 proteasome regulator subunits P28α and P28β.22 These changes result in the exclusive production of iP in stimulated DCs.
Our data presented in Figure 2 using an HLA-B51–restricted clone specific for a peptide generated exclusively through degradation of the ubiquitously expressed RU1 self-protein by the cP and not the iP, provides functional confirmation that iDCs express the cP, and that this cP function is rapidly lost after the induction of DC maturation. Simply by inhibiting the expression of the inducible iP subunits using siRNA transfection immediately before the induction of maturation, cP functional activity in the mDC was restored.
The effect of iPsiRNA on DC proteasome function, peptide presentation, and the induction of immune responses in each of our experiments was observed within 24 hours of transfection. Because the half-life of assembled mammalian proteasomes has been reported to be 5 days,23,24 our data suggests that it is newly synthesized proteasomes that are critical for generating peptides presented in the context of HLA class I for the induction of immunity. The functional changes in peptide presentation we observed occurred despite the fact that the overall intracellular proteasome composition was only moderately shifted from iP to cP after transfection of DCs with iPsiRNA (Figure 1B,C).
Since the iP has been demonstrated to associate with the endoplasmic reticulum (ER) and class I peptide loading occurs in close association with the ER membrane,25,26 it may be that newly synthesized proteasomes are most closely associated with the ER and involved in antigen processing.16 Experiments currently under way in our laboratory in which monocytes are transfected with iPsiRNA and then differentiated into DCs using IL-4 and GM-CSF show that these DCs have a more pronounced shift in intracellular proteasomes from the iP to the cP (data not shown). Experiments to evaluate antigen processing and the immunogenicity of these DCs are in progress.
Results of our in vitro melanoma immunotherapy model (Figures 5,Figure 6–7) clearly support the concept that the use of mature TAA-loaded DC stimulators expressing the normal iP may be “mistargeting” antitumor immune responses. As shown for all 3 “patients,” the maximal antigen-specific immune response, be it against autologous TAA RNA–transfected DCs or, most important, against autologous melanoma cells, was always enhanced when the iP of the stimulator DC was inhibited by iPsiRNA transfection.
DCs loaded with iP-generated peptides have clearly been shown to stimulate antitumor immunity. Since some antigenic peptides are generated by both the cP and the iP, albeit at different quantitative levels, low levels of some “iP-generated” peptides generated by the cP may be presented on target tumor cells and recognized by CTLs specific for the “iP-generated” peptide. Such will not be the case for all iP-generated peptides, particularly those destroyed by the cP.18
By exogenously loading mDCs with cP- or iP-generated peptides, any peptide dose limitations for CTL induction are overcome, and peptide-specific CTLs can be stimulated. CTLs induced with iP-generated peptide-loaded DCs might also kill tumor cells that express above-threshold levels of specific “iP-generated” peptide generated at low levels by the cP. In contrast, when DCs are loaded with tumor cell lysates, tumor proteins, or TAA-encoding RNA as a source of tumor antigens, antigenic protein processing by the DC proteasome will determine which peptides are presented for the induction of a CTL response, and whether the critical threshold for CTL induction is reached. By both inhibiting iP expression and concomitantly enhancing cP expression in mDCs using iPsiRNA transfection, we appear to have been able to surpass this threshold for cP-generated peptide epitope presentation and induce more effective antitumor CTL activity in our in vitro melanoma immunotherapy model.
In a variety of clinical immunotherapy trials, immune responses against defined melanoma antigens have been demonstrated. Recently, Rosenberg and associates reported a trial in which patients with metastatic melanoma received injections of ex vivo expanded autologous T cells retrovirally transduced with T-cell receptors (TCRs) specific for TAA-derived peptides expressed in the context of HLA-A2, where 2 of 15 patients demonstrated partial clinical responses.27 Interestingly, 2 of the 4 TCRs used for that study were specific for A2-restricted peptides that have been shown to be generated exclusively by the iP and not the cP (Mart27-3512 and gp100209-21718 ), and were used to assess CTL responses in our study (Figure 4A). It is possible that adoptive transfer of T cells with TCRs specific for these iP-generated TAA peptides might not have optimally targeted melanoma cells presenting TAA-derived peptides generated by the cP.
Using peptide antigen–loaded DCs, Nestle et al vaccinated patients with melanoma using intranodal injection and noted both objective immunologic responses as well as subjective clinical improvement in some patients.28 Although a variety of additional DC-based immunotherapy trials for the treatment of melanoma are currently in progress, almost all of these studies use DCs loaded with defined HLA-restricted peptides, an approach that is limited to patients of certain HLA types and is restricted to tumor-antigenic peptides that have previously been identified.
To overcome these limitations, we envision using mDCs loaded not with peptides, but with tumor RNA or tumor cell lysates for immunotherapy. This would expand the application of DC-based immunotherapy to patients of any HLA type and with melanomas or other tumors not necessarily expressing defined tumor antigens with known HLA-restricted epitopes. In such cases, processing of the tumor-antigenic proteins by the proteasome is critical for the generation of immunogenic peptides presented in the context of HLA class I by DCs. By altering the DC proteasome from the iP to the cP using iPsiRNA transfection we have demonstrated that mDCs process tumor-antigenic proteins into peptides that may be more relevant for the induction of effective antimelanoma immunity. Such an ability to enhance the stimulation of antimelanoma immunity would benefit patients with metastatic melanoma as well as other malignancies, and would not be limited by patient HLA type.
In conclusion, our studies suggest that by altering tumor antigen–loaded DCs to favor the presentation of peptides generated by the cP rather than the iP, such DCs might be superior immunogens for tumor immunotherapy. A clinical immunotherapy trial using iPsiRNA-transfected DCs in patients with metastatic melanoma is currently being initiated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Kent Weinhold for his scientific guidance, Dr B. J. Van den Eynde for generously providing the RU1 peptide-specific CTL clone and RCC cell line, Dr Soldano Ferrone for helpful suggestions as well as flow cytometry reagents, Dr Robert Lefkowitz and Diane Sawyer for assistance with the metabolic labeling experiments, and Drs Paul Kuo and Danny Jacobs of the Duke University Department of Surgery for additional research support.
This work was supported by a Veterans Administration Merit Review Grant.
Authorship
Contribution: J.D. designed and performed research; D.-T.L., R.H., W.Q., and G.H. performed research; H.S. contributed vital new reagents; D.S.T. analyzed data; and S.K.P. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Scott K. Pruitt, Box 3966, Duke University Medical Center, Durham, NC 27710; e-mail:scott.pruitt@duke.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal