Antiphospholipid syndrome (APS) is an autoimmune prothrombotic disorder associated with autoantibodies to phospholipid (PL)–binding proteins, such as β2-glycoprotein I (β2GPI). We have recently reported that binding of β2GPI to anionic PL facilitates processing and presentation of the cryptic β2GPI epitope that activates pathogenic autoreactive T cells. To clarify mechanisms that induce sustained presentation of the dominant antigenic β2GPI determinant in patients with APS, T-cell proliferation induced by β2GPI-treated phosphatidylserine liposome (β2GPI/PS) was evaluated in bulk peripheral blood mononuclear cell cultures. T cells from patients with APS responded to β2GPI/PS in the presence of immunoglobulin G (IgG) anti-β2GPI antibodies derived from APS plasma, and this response was completely inhibited either by the depletion of monocytes or by the addition of anti-FcγRI antibody. These findings indicate that efficient presentation of the cryptic determinants can be achieved by monocytes undergoing FcγRI-mediated uptake of β2GPI-bound anionic surfaces in the presence of IgG anti-β2GPI antibodies. Finally, β2GPI-bound oxidized LDL or activated platelets also induced the specific T-cell response. Continuous exposure to these anionic surfaces may play a critical role in maintaining the pathogenic anti-β2GPI antibody response in patients with APS.
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by arterial and venous thrombosis as well as recurrent intrauterine fetal loss in the presence of antiphospholipid antibodies.1 β2-glycoprotein I (β2GPI) is the most common antigenic target recognized by the antiphospholipid antibodies, and anti-β2GPI antibodies are shown to be strongly associated with thrombosis and other clinical manifestations of APS.2,–4 β2GPI is a plasma protein that binds various anionic substances, including phospholipids (PLs), lipoproteins, and activated platelets and endothelial cells.5,–7 Several lines of evidence accumulated from animal models suggest that anti-β2GPI antibodies are directly involved in the pathogenic processes of APS.8,9
We have recently identified CD4+ T cells responsive to β2GPI in patients with APS.10,–12 β2GPI-reactive T cells can promote production of pathogenic immunoglobulin G (IgG) anti-β2GPI antibodies from autologous B cells in vitro. These T cells respond to bacterially expressed recombinant β2GPI fragments and chemically reduced β2GPI, but fail to respond to native β2GPI,10 indicating that the epitopes recognized by β2GPI-reactive T cells are cryptic determinants that are not generated through processing of native β2GPI under normal circumstances. One of the major cryptic determinants recognized by β2GPI-reactive T cells is the region spanning amino acids (AAs) 276-290, which contains the major PL-binding site at AA 281-288,13,14 in the context of HLA-DRB4*0103 (DR53).11 In our recent study employing β2GPI-reactive CD4+ T-cell clones generated from patients with APS, dendritic cells or macrophages pulsed with β2GPI-bound phosphatidylserine (PS) liposome induced a response of T-cell clones specific for a peptide encoding AA 276–290 (p276-290) in HLA-DR–restricted and antigen-processing–dependent manners. In contrast, those pulsed with β2GPI or PS liposome alone failed to induce a response.15 Together these findings indicate that specialized antigen-presenting cells (APCs) capturing β2GPI-coated anionic PLs efficiently present a disease-relevant cryptic T-cell determinant of β2GPI as a result of antigen processing.
In patients with APS, anti-β2GPI antibody levels are usually stable for many years. However, it remains unclear what mechanisms are responsible for the sustained presentation of the dominant cryptic β2GPI determinant that activates β2GPI-reactive T cells to subsequently produce pathogenic anti-β2GPI antibodies. To elucidate these mechanisms, we examined the cellular and molecular factors required for the sustained activation of β2GPI-reative T cells in patients with APS.
Patients, materials, and methods
Patients and controls
This study examined 5 patients, and all fulfilled the revised Sapporo criteria for APS proposed by the International Workshop.16 These patients were selected based on the presence of DRB4*0103 (DR53), which is known to present a p276-290 peptide to T cells,11 and positive IgG anti-β2GPI antibody. The HLA class II alleles, including DRB1 and DRB4, were determined by restriction fragment length polymorphisms combined with locus-specific polymerase chain reaction using peripheral blood granulocyte-derived genomic DNA as a template.17 IgG anti-β2GPI antibody levels were measured with a commercial enzyme-linked immunosorbent assay (ELISA) kit (Yamasa, Choshi, Japan) using immobilized β2GPI-cardiolipin complex as an antigen source. A commercial kit based on Russell viper venom test (Gradipore, Sydney, Australia) was used to determine the presence of lupus anticoagulant. At the time of blood examination, all the patients were taking low-dose corticosteroids (< 10 mg/day) and low-dose aspirin. Peripheral blood from healthy volunteers was also used as a control source of plasma. All samples were obtained after the patients and control subjects gave their written informed consent in accordance with the Declaration of Helsinki. The study protocol was approved by Keio University International Review Board.
Antigen preparations
Human β2GPI was purified from normal pooled plasma,18 and reduced β2GPI was prepared by incubating β2GPI with dithiothreitol as previously described.10 We generated a panel of recombinant maltose-binding protein (MalBP) fusion proteins expressing full-length β2GPI (GP-F), domains I and II (GP1), domains III and IV (GP2), and domains IV and V (GP3).10 MalBP alone was prepared as a control antigen. Two 15-mer peptides, p276-290 and a peptide encoding AA 306–320 of human β2GPI (p306-320), were synthesized using a solid-phase multiple synthesizer (Advanced ChemTech, Louisville, KY).11
Liposome containing bovine brain-derived PS (Sigma, St Louis, MO), with a composition of dioleoylphosphatidylcholine (Avanti Polar Lipids, Alabaster, AL) at a molar ratio of 3:7, was prepared and adjusted to a final concentration of 1 μmol/mL.19,20 Low density lipoprotein (LDL) was isolated from freshly prepared normal human plasma by ultracentrifugation, and oxidized LDL (oxLDL) was prepared by incubating LDL with 5 μM CuSO4 for 8 hours at 37°C.20 LDL and oxLDL were adjusted to 100 μg/mL of apoB equivalent. Human platelets were separated from platelet-rich plasma using a modified gel filtration method21 to minimize their activation during an isolation procedure. Resting platelets were then activated by incubation with bovine thrombin (1 U/mL; Mochida, Tokyo, Japan) for 15 minutes. All preparations were incubated with or without native β2GPI (100 μg/mL) for 30 minutes at room temperature immediately prior to use in the cultures.
Cell preparations
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by Lymphoprep (Fresenius Kabi Norge AS, Oslo, Norway) density-gradient centrifugation. In some experiments, PBMCs were depleted of CD14+ monocytes or CD19+ B cells by incubation with anti-CD14 or anti-CD19 monoclonal antibody (mAb)–coupled magnetic beads (Miltenyi Biotecch, Bergisch Gladbach, Germany), respectively, followed by magnetic cell sorting column separation according to the manufacturer's protocol.
Preparation and depletion of IgG from plasma
The IgG fraction was purified or depleted from plasma samples using HiTrap protein G (Amersham Biosciences, Uppsala, Sweden) as described previously.22 Purity of IgG fractions was confirmed to be more than 95% by sodium dodecyl sulfate–polyacrylamide gel electropheresis, followed by densitometry on Coomassie blue–stained gels. In some experiments, purified IgG was treated with pepsin to prepare F(ab′)2 using a Fab2 preparation kit (Pierce Biotechnology, Rockford, IL). We also prepared IgG fractions depleted of antibodies specific to β2GPI. Briefly, purified IgG samples were treated 3 times with cardiolipin-coated 96-well immunoplates (Nunc F96Maxisorp, Roskilde, Denmark), which were preincubated with β2GPI or phosphate-buffer saline for 30 minutes. The supernatants were then collected as anti-β2GPI antibody–depleted or mock-treated IgG. Removal of anti-β2GPI antibody was confirmed by complete loss of antibody reactivity on the anti-β2GPI antibody ELISA.
Assays for antigen-specific T-cell response
Antigen-specific T-cell proliferation in the primary cultures was assayed as described previously10 with some modifications. Briefly, PBMCs (105/well) were cultured with or without antigen in 96-well flat-bottomed culture plates for 7 days. RPMI 1640 supplemented with either 10% fetal bovine serum (FBS; JRH Bioscience, Lenexa, KS) or 8% platelet-poor plasma, which was derived from patients with APS and healthy donors, was used as medium. Prior to use, FBS and plasma samples were heat-inactivated and depleted of β2GPI by passing the samples through a HiTrap Heparin column (Amersham Biosciences) twice, to eliminate the potential influence of intrinsic β2GPI on the generation of the antigenic peptides. 3H-thymidine (0.5 μCi [0.0185 MBq]/well) was added to the cultures during the final 16 hours. The cells were harvested, and 3H-thymidine incorporation was measured in a Top-Count microplate scintillation counter (Packard, Meriden, CT). Native β2GPI, reduced β2GPI, GP-F, GP1, GP2, GP3, and MalBP were used as antigens at a concentration of 10 μg/mL. In addition, PS liposome (0.1 μmol/mL), LDL, oxLDL (10 μg/mL apoB equivalent), resting platelets, or activated platelets (106/well) were added to the cultures, with or without preincubation with β2GPI. To exclude nonspecific unresponsiveness of T cells, all experiments included a culture with phytohemagglutinin at a final concentration of 1 μg/mL. In some experiments, purified IgG, F(ab′)2, or anti-β2GPI antibody–depleted or mock-treated IgG was added at the initiation of the culture. Anti-FcγRI (clone 10.1; R&D Systems, Minneapolis, MN), anti-HLA-DR (clone L243; Leinco Technologies, Baldwin, MO), or isotype-matched control mAb was also added to the culture at a final concentration of 2.5 μg/mL. All experiments were carried out in duplicate or triplicate, and the values are the mean counts per minute (cpm) plus or minus the standard deviation of multiple determinations. In some instances, a T-cell response specific to β2GPI-treated PS liposome (β2GPI/PS) was expressed as the ratio of cpm in the culture with β2GPI/PS to cpm in the culture with PS liposome alone.
Secondary stimulation of peripheral blood T cells was also performed as described.10 PBMCs were primed with β2GPI/PS in medium supplemented with 8% autologous plasma for 10 days. Viable cells were then cultured for an additional 3 days in the presence of 50 U/mL recombinant interleukin-2 (Biogen Idec, San Diego, CA) and irradiated (3000 rad) autologous monocyte-derived dendritic cells in medium supplemented with 10% FBS in the absence or presence of β2GPI, reduced β2GPI, GP-F, GP1, GP2, GP3, MalBP (10 μg/mL), p276-290, or p306-320 (5 μg/mL). Frequencies of β2GPI-reactive T cells in peripheral blood T cells were estimated by limiting dilution analysis using GP-F as an antigen.23 The recognition of p276-290 by peripheral blood T cells was determined based on the specific response to p276-290 by at least 2 T-cell clones established by repeated stimulation of peripheral blood T cells with GP-F.11
Results
Clinical and immunologic characteristics of patients with APS
As shown in Table 1, all patients with APS had thrombosis and/or loss of pregnancy, and were positive for lupus anticoagulant. IgG anti-β2GPI antibody titer was high in all but one patient (APS1). Frequencies of β2GPI-reactive T cells were variable among patients, and ranged from 2.9 to 12.4 per 104 peripheral blood T cells. In addition, T-cell recognition of p276-290 was detected in all 3 patients examined.
Clinical and immunologic characteristics of patients with APS analyzed in this study
| Patient no. . | Age/sex . | Thrombosis . | Loss of pregnancy . | IgG anti-β2GPI antibodies (U/mL)† . | HLA class II alleles: DRB1 . | Frequency of β2GPI-reactive T cells in circulation/104 T cells . | Recognition of p276–290 by peripheral blood T cells . |
|---|---|---|---|---|---|---|---|
| APS1 | 51/F | None | + | 16 | *1502/*0405 | 4.5 | NT |
| APS2 | 43/F | DVT, stroke | + | >120 | *0405/*1202 | 2.9 | NT |
| APS3 | 46/F | DVT, PE, retinal artery thrombosis | + | >120 | *1502/*0901 | 6.8 | + |
| APS4 | 47/F | Stroke | + | >120 | *1501/*0403 | 8.1 | + |
| APS9 | 46/F | DVT, PE, stroke, amaurosis fugax | NA | >120 | *0901 | 12.4 | + |
| Patient no. . | Age/sex . | Thrombosis . | Loss of pregnancy . | IgG anti-β2GPI antibodies (U/mL)† . | HLA class II alleles: DRB1 . | Frequency of β2GPI-reactive T cells in circulation/104 T cells . | Recognition of p276–290 by peripheral blood T cells . |
|---|---|---|---|---|---|---|---|
| APS1 | 51/F | None | + | 16 | *1502/*0405 | 4.5 | NT |
| APS2 | 43/F | DVT, stroke | + | >120 | *0405/*1202 | 2.9 | NT |
| APS3 | 46/F | DVT, PE, retinal artery thrombosis | + | >120 | *1502/*0901 | 6.8 | + |
| APS4 | 47/F | Stroke | + | >120 | *1501/*0403 | 8.1 | + |
| APS9 | 46/F | DVT, PE, stroke, amaurosis fugax | NA | >120 | *0901 | 12.4 | + |
All patients were lupus anticoagulant positive; all DRB4 alleles were *0103.
DVT indicates deep venous thrombosis of lower extremity; PE, pulmonary embolism; NA, not applicable; and NT, not tested.
Normal range less than 3.5 U/mL.
T-cell response induced by β2GPI/PS in PBMC cultures
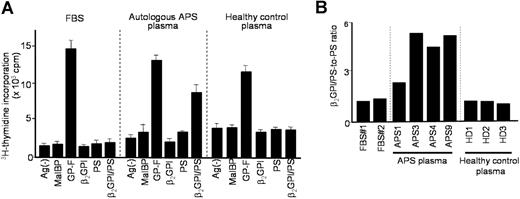
We first examined the responses of peripheral blood T cells to β2GPI/PS using regular medium supplemented with FBS (Figure 1A). T cells from all 5 patients responded to GP-F, but failed to proliferate in the presence of β2GPI/PS. Interestingly, a T-cell response to β2GPI/PS, as well as to GP-F, was detected when a patient's autologous plasma was used instead of FBS to supplement the culture medium. This response was blocked by anti–HLA-DR mAb, but not by control mAb (data not shown). However, a β2GPI/PS-induced response was not detected in the culture with allogenic plasma from a healthy individual. This finding was reproducible in a total of 7 PBMC samples obtained from 5 patients with APS.
T-cell response to β2GPI/PS in bulk PBMC cultures supplemented with FBS, autologous APS plasma, or healthy control plasma. (A) PBMCs from APS4 were cultured in triplicate with or without antigens, including MalBP, GP-F, β2GPI, PS, and β2GPI/PS, in medium supplemented with FBS, autologous APS plasma, or healthy control plasma. The antigen-induced T-cell proliferative response was assessed by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of triplicate measurements. Analogous findings were obtained in 7 independent experiments in PBMCs from all 5 patients with APS. (B) β2GPI/PS-specific T-cell response in PBMC cultures of APS4 in medium supplemented with 2 different lots of FBS (no. 1 and no. 2), plasma samples from 4 APS patients (APS1, 3, 4, and 9), or plasma samples from 3 healthy donors (HD1, 2, and 3). β2GPI/PS-specific T-cell response was expressed as a β2GPI/PS-to-PS ratio, which was the mean cpm incorporated in the triplicate culture with β2GPI/PS divided by the mean cpm incorporated in the triplicate culture with PS alone (standard deviations for the individual results were within 20% of the mean in all cases). Similar results were obtained from 3 additional patients with APS (APS1, APS3, and APS9).
T-cell response to β2GPI/PS in bulk PBMC cultures supplemented with FBS, autologous APS plasma, or healthy control plasma. (A) PBMCs from APS4 were cultured in triplicate with or without antigens, including MalBP, GP-F, β2GPI, PS, and β2GPI/PS, in medium supplemented with FBS, autologous APS plasma, or healthy control plasma. The antigen-induced T-cell proliferative response was assessed by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of triplicate measurements. Analogous findings were obtained in 7 independent experiments in PBMCs from all 5 patients with APS. (B) β2GPI/PS-specific T-cell response in PBMC cultures of APS4 in medium supplemented with 2 different lots of FBS (no. 1 and no. 2), plasma samples from 4 APS patients (APS1, 3, 4, and 9), or plasma samples from 3 healthy donors (HD1, 2, and 3). β2GPI/PS-specific T-cell response was expressed as a β2GPI/PS-to-PS ratio, which was the mean cpm incorporated in the triplicate culture with β2GPI/PS divided by the mean cpm incorporated in the triplicate culture with PS alone (standard deviations for the individual results were within 20% of the mean in all cases). Similar results were obtained from 3 additional patients with APS (APS1, APS3, and APS9).
Next, PBMCs from a patient with APS were cultured with β2GPI/PS or PS liposome alone in medium supplemented with 2 different lots of FBS, plasma samples from 4 patients with APS, or samples from 3 healthy donors (Figure 1B). The β2GPI/PS-specific response was exclusively detected in cultures with autologous and allogenic plasmas derived from patients with APS, although the degree of response was variable among APS plasmas. Analogous findings were obtained with PBMCs from 3 additional patients with APS. In all cases, the lowest response was detected in the culture supplemented with APS1 plasma, which contained low-titer anti-β2GPI antibodies.
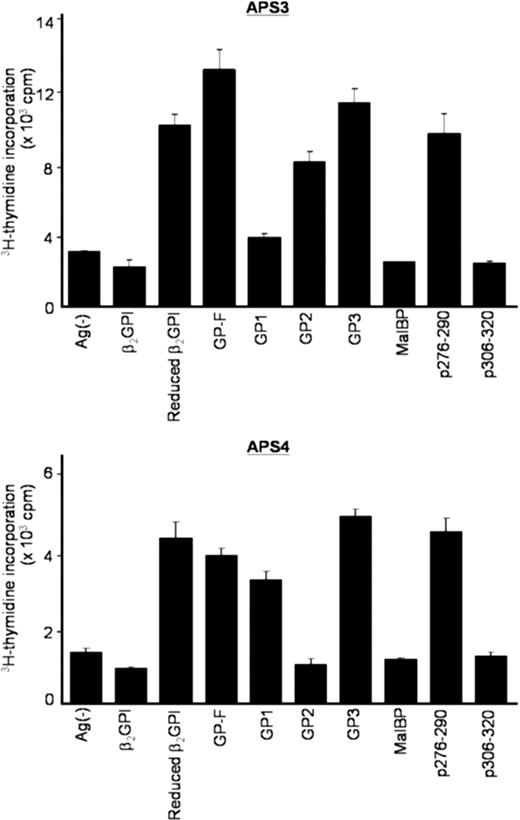
We next sought to confirm whether T-cell responses induced by β2GPI/PS in cultures with APS plasma were specific to β2GPI. Peripheral blood T cells primed with β2GPI/PS in medium supplemented with autologous plasma were further examined for their reactivity to various β2GPI preparations in the secondary culture with FBS (Figure 2). β2GPI/PS-primed T cells from all 5 patients specifically responded to reduced β2GPI, GP-F, and GP3, indicating a specific recognition of β2GPI-derived peptides. More important, the cryptic p276-290 was efficiently presented by APCs in culture with β2GPI/PS and APS plasma. T-cell recognition of GP1 was detected in APS2, APS4, and APS9 samples, whereas recognition of GP2 was detected in APS3 and APS9. Taken together, these findings together indicate that a soluble factor(s) contained in plasma from patients with APS, but not in FBS or plasma from healthy individuals, plays an essential role in activation of β2GPI-specific T cells in bulk PBMC cultures with β2GPI/PS.
Proliferative responses of β2GPI/PS-primed T cells to various β2GPI preparations in secondary cultures. PBMCs from APS3 (top) and APS4 (bottom) were stimulated with β2GPI/PS for 10 days in medium supplemented with autologous plasma. The viable T cells were then cultured in duplicate with β2GPI, reduced β2GPI, GP-F, GP1, GP2, GP3, MalBP, p276-290, or p306-320 in medium containing FBS. After 3 days, 3H-thymidine incorporation was measured. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements.
Proliferative responses of β2GPI/PS-primed T cells to various β2GPI preparations in secondary cultures. PBMCs from APS3 (top) and APS4 (bottom) were stimulated with β2GPI/PS for 10 days in medium supplemented with autologous plasma. The viable T cells were then cultured in duplicate with β2GPI, reduced β2GPI, GP-F, GP1, GP2, GP3, MalBP, p276-290, or p306-320 in medium containing FBS. After 3 days, 3H-thymidine incorporation was measured. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements.
IgG anti-β2GPI autoantibody as an essential factor for T-cell recognition of β2GPI/PS
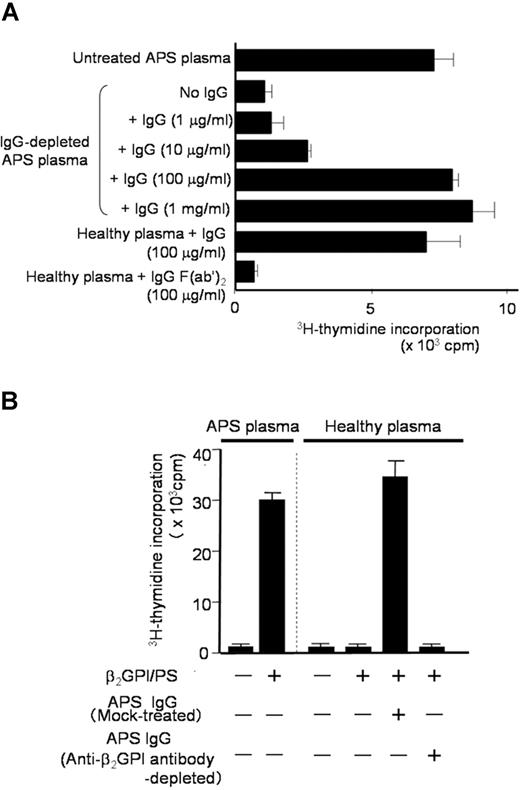
Since the degree of the β2GPI/PS-specific T-cell response appeared to correlate with IgG anti-β2GPI antibody titers, we hypothesized that IgG anti-β2GPI antibodies in APS plasma are required for peripheral blood T cells to respond to β2GPI/PS. To test this hypothesis, we first prepared IgG-depleted APS plasma samples to evaluate the β2GPI/PS-induced T-cell response (Figure 3A). Depletion of IgG from APS plasma resulted in complete loss of the β2GPI/PS-induced T-cell response, but addition of autologous IgG back to the IgG-depleted APS plasma restored the response in a dose-dependent fashion. In contrast, addition of IgG prepared from healthy plasma had no effect (data not shown). Interestingly, β2GPI/PS-induced T-cell response was also detected in medium supplemented with healthy plasma in the presence of IgG derived from APS plasma. This response was abolished when F(ab′)2 was used instead of intact IgG, indicating an important role of the Fc portion of IgG.
β2GPI/PS-induced T-cell response in PBMC cultures with or without IgG derived from APS plasma. (A) PBMCs obtained from APS3 were cultured in triplicate with β2GPI/PS in medium supplemented with untreated or IgG-depleted autologous APS plasma, or healthy plasma. Purified IgG (1 μg/mL-1 mg/mL) or IgG F(ab′)2 (100 μg/mL) from APS3 was added to the cultures. After 7 days, the T-cell proliferative response induced by β2GPI/PS was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar). Concordant results were obtained with a sample from APS4. (B) PBMCs derived from APS3 were cultured in triplicate with or without β2GPI/PS in medium supplemented with autologous APS plasma or healthy plasma. An anti-β2GPI antibody–depleted or mock-treated autologous IgG fraction was added to the initiation of cultures. After 7 days, the T-cell proliferative response was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar). Concordant results were obtained with a sample from APS4.
β2GPI/PS-induced T-cell response in PBMC cultures with or without IgG derived from APS plasma. (A) PBMCs obtained from APS3 were cultured in triplicate with β2GPI/PS in medium supplemented with untreated or IgG-depleted autologous APS plasma, or healthy plasma. Purified IgG (1 μg/mL-1 mg/mL) or IgG F(ab′)2 (100 μg/mL) from APS3 was added to the cultures. After 7 days, the T-cell proliferative response induced by β2GPI/PS was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar). Concordant results were obtained with a sample from APS4. (B) PBMCs derived from APS3 were cultured in triplicate with or without β2GPI/PS in medium supplemented with autologous APS plasma or healthy plasma. An anti-β2GPI antibody–depleted or mock-treated autologous IgG fraction was added to the initiation of cultures. After 7 days, the T-cell proliferative response was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar). Concordant results were obtained with a sample from APS4.
We further examined the effects of depletion of β2GPI-specific antibody on the β2GPI/PS-induced T-cell response in PBMC cultures with APS IgG (Figure 3B). β2GPI/PS-induced T-cell response was detected in the presence of mock-treated APS IgG, but completely abolished by depletion of β2GPI-reactive IgG. These findings indicate that IgG anti-β2GPI antibodies are required for the T cells of patients with APS to respond to β2GPI/PS in bulk PBMC cultures.
Roles of β2GPI/PS-containing immune complex in β2GPI/PS-induced T-cell response
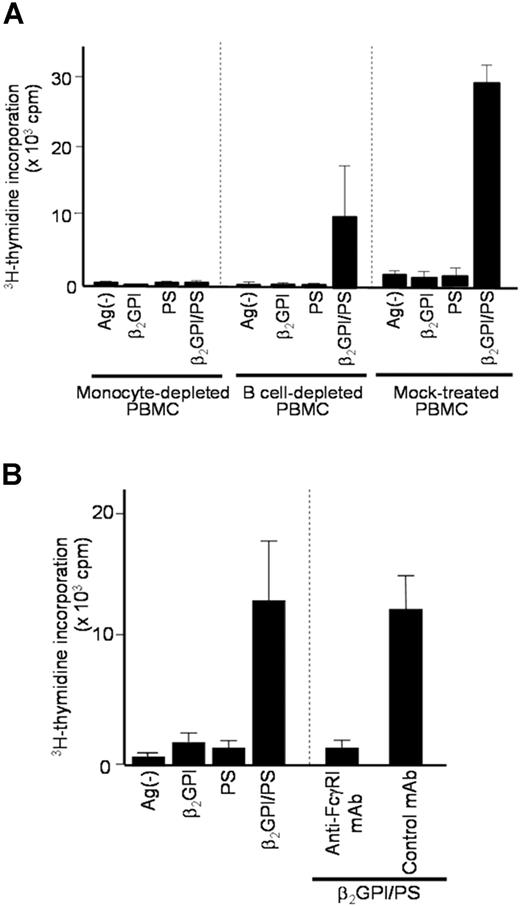
Since anti-β2GPI antibodies in sera from patients with APS recognize β2GPI/PS,20 it is likely that β2GPI/PS is readily opsonized by IgG anti-β2GPI antibodies in culture with APS plasma. To evaluate which APCs contained in PBMCs capture this immune complex to induce a specific T-cell response to β2GPI peptides, we analyzed PBMCs depleted of CD14+ monocytes, CD19+ B cells, or mock-treated in cultures with β2GPI/PS and autologous plasma (Figure 4A). The β2GPI/PS-induced T-cell response was completely inhibited by depletion of monocytes, but was partially suppressed by depletion of B cells, suggesting a primary role of monocytes in our system.
Effects of APC depletion or anti-FcγRI mAb on β2GPI/PS-induced T-cell response. (A) CD14+ monocyte-depleted, CD19+ B-cell–depleted, and mock-treated PBMCs derived from APS3 were cultured for 7 days with or without β2GPI, PS, or β2GPI/PS in medium supplemented with autologous APS plasma, and the T-cell proliferative response was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements. Analogous results were obtained in a total of 4 independent experiments using samples from 3 patients with APS (APS1, APS3, and APS4). (B) PBMCs from APS2 were cultured for 7 days with or without β2GPI, PS, or β2GPI/PS in medium supplemented with autologous APS plasma. Anti-FcγRI or isotype-matched control mAb was added to the initiation of cultures. The T-cell proliferative response was evaluated by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements. Concordant results were obtained with samples from 3 patients with APS (APS2, APS3, and APS9).
Effects of APC depletion or anti-FcγRI mAb on β2GPI/PS-induced T-cell response. (A) CD14+ monocyte-depleted, CD19+ B-cell–depleted, and mock-treated PBMCs derived from APS3 were cultured for 7 days with or without β2GPI, PS, or β2GPI/PS in medium supplemented with autologous APS plasma, and the T-cell proliferative response was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements. Analogous results were obtained in a total of 4 independent experiments using samples from 3 patients with APS (APS1, APS3, and APS4). (B) PBMCs from APS2 were cultured for 7 days with or without β2GPI, PS, or β2GPI/PS in medium supplemented with autologous APS plasma. Anti-FcγRI or isotype-matched control mAb was added to the initiation of cultures. The T-cell proliferative response was evaluated by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements. Concordant results were obtained with samples from 3 patients with APS (APS2, APS3, and APS9).
We further evaluated the potential involvement of Fcγ receptors in recognition of the immune complex by monocytes, as the anti-β2GPI F(ab′)2 was incapable of inducing the T-cell response to β2GPI/PS. The β2GPI/PS-induced T-cell response was completely blocked by anti-FcγRI mAb, but not by control mAb (Figure 4B). Together these findings indicate that efficient β2GPI/PS-induced T-cell response is achieved by monocytes undergoing FcγRI-mediated uptake of β2GPI/PS opsonized by IgG anti-β2GPI autoantibodies.
T-cell response to β2GPI-treated oxLDL and platelet microparticles
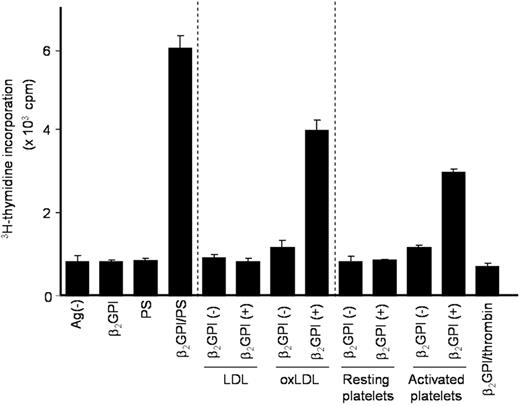
PS liposomes were chemically synthesized, and may not be relevant to patients with APS in vivo. To examine whether anionic substances present in the circulation, such as oxLDL or platelet microparticles, can substitute for PS liposomes in inducing the β2GPI-specific T-cell response, PBMCs from a representative patient with APS were cultured with various anionic and control substances pretreated with or without β2GPI in medium supplemented with autologous plasma (Figure 5). OxLDL or activated platelets pretreated with β2GPI induced a T-cell proliferative response, as observed in cultures with β2GPI/PS. These responses were specifically inhibited by anti–HLA-DR mAb (data not shown). Thus, oxLDL and activated platelets can be in vivo sources of anionic surfaces that bind β2GPI and promote the efficient presentation of β2GPI cryptic peptides by APCs.
T-cell responses to β2GPI-treated anionic substances present in circulation. PBMCs from APS4 were cultured with or without various antigen preparations in medium supplemented with autologous APS plasma. Antigens used included β2GPI alone, as well as PS, LDL, oxLDL, resting platelets, and activated platelets, which were treated either with or without β2GPI. Thrombin, which was used to activate platelets, in combination with β2GPI served as a control. T-cell proliferative response was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements. Analogous results were obtained in samples from all 5 patients with APS.
T-cell responses to β2GPI-treated anionic substances present in circulation. PBMCs from APS4 were cultured with or without various antigen preparations in medium supplemented with autologous APS plasma. Antigens used included β2GPI alone, as well as PS, LDL, oxLDL, resting platelets, and activated platelets, which were treated either with or without β2GPI. Thrombin, which was used to activate platelets, in combination with β2GPI served as a control. T-cell proliferative response was measured by 3H-thymidine incorporation. Results are shown as mean (column) and standard deviation (error bar) of duplicate measurements. Analogous results were obtained in samples from all 5 patients with APS.
Discussion
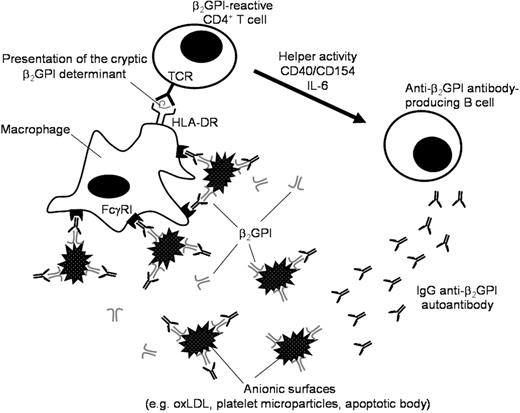
This study evaluated the potential cellular and molecular mechanisms that induce sustained presentation of the dominant cryptic β2GPI determinant that activates β2GPI-reactive T cells to subsequently produce pathogenic anti-β2GPI antibodies in patients with APS. Here we demonstrate that efficient presentation of cryptic determinants recognized by β2GPI-reactive T cells is achieved by monocytes undergoing FcγRI-mediated uptake of β2GPI/PS opsonized by IgG anti-β2GPI antibodies. High avidity IgG anti-β2GPI antibodies, which were reported to possess high pathogenicity,24 would also have enhanced capacity to promote this process. We propose a model by which a pathogenic loop maintains sustained anti-β2GPI autoantibody production in patients with APS (Figure 6). This model consists of 3 major players: β2GPI-reactive CD4+ T cells, anti-β2GPI antibody-producing B cells, and macrophages. Upon recognition of β2GPI cryptic peptides, such as p276-290, presented by macrophages in the context of HLA-DR, β2GPI-reactive CD4+ T cells are activated and exert helper activity that induces IgG anti-β2GPI antibody production from B cells. This process can be achieved by T-B cell collaboration through CD40-CD154 engagement and T cell–derived IL-6.11 IgG anti-β2GPI antibodies subsequently recognize β2GPI-bound anionic surfaces in circulation, resulting in enhanced phagocytosis of this immune complex by macrophages through FcγRI. In this regard, it has been shown that anti-β2GPI antibodies in APS sera are predominantly of IgG2 subclass,25,26 which has low affinity to FcγRI. However, anti-β2GPI antibodies of IgG1 or IgG3 subclass were also detected in many patients with APS. These low levels of anti-β2GPI antibodies with high binding affinity to FcγRI may be sufficient to drive the pathogenic loop. We have previously shown that β2GPI binding to anionic substances promotes the generation of β2GPI cryptic peptides by protecting the major PL-binding site from protease attack during antigen-processing by dendritic cells or macrophages.15 Since it has been shown that antibody binding to the antigen boosts the generation of some minor epitopes,27 binding of IgG anti-β2GPI antibodies to the β2GPI-anionic substance complex may further amplify generation of previously cryptic β2GPI peptides. Moreover, this immune complex is likely to stimulate monocytes via FcγRI to secrete tissue factor, which is shown to play an important role in thrombus formation in patients with APS.28 Partial suppression of the β2GPI/PS-induced T-cell response by depletion of B cells in our system suggests that presentation of cryptic β2GPI peptides could be mediated through B cells that capture β2GPI/PS via specific B-cell receptors. This process, however, might have less of an impact on the T-cell response, due likely to low abundance of specific B cells recognizing β2GPI/PS. The mechanism that triggers anti-β2GPI antibody response in patients with APS remains unclear, but once this autoimmune loop is established, pathogenic anti-β2GPI antibodies are continuously produced.
A schematic model representing a continuous autoimmune loop carried out by macrophage, β2GPI-reactive CD4+ T cell, and anti-β2GPI antibody–producing B cell. The macrophage efficiently presents the cryptic β2GPI determinant in the context of HLA-DR. The β2GPI-reactive CD4+ T cell is activated by recognition of the cryptic β2GPI peptide and exerts helper activity that induces production of IgG anti-β2GPI autoantibodies from the specific B cell. The immune complex consisting of anionic surfaces, β2GPI, and IgG anti-β2GPI antibodies were captured by macrophages via FcγRI.
A schematic model representing a continuous autoimmune loop carried out by macrophage, β2GPI-reactive CD4+ T cell, and anti-β2GPI antibody–producing B cell. The macrophage efficiently presents the cryptic β2GPI determinant in the context of HLA-DR. The β2GPI-reactive CD4+ T cell is activated by recognition of the cryptic β2GPI peptide and exerts helper activity that induces production of IgG anti-β2GPI autoantibodies from the specific B cell. The immune complex consisting of anionic surfaces, β2GPI, and IgG anti-β2GPI antibodies were captured by macrophages via FcγRI.
The presence of anionic substances with the capacity to bind β2GPI is essential to drive the pathogenic loop inducing continuous anti-β2GPI antibody production in patients with APS. Potential anionic substances in the circulation include apoptotic bodies, microparticles derived from activated platelets and endothelial cells, and oxLDL. Since β2GPI is abundantly present in the circulation (∼200 μg/mL), excessive exposure to anionic substances would result in the immediate formation of a complex with β2GPI. In the present study, we have clearly demonstrated that microparticles derived from activated platelets and oxLDL can function as a substitute for the PS liposome that binds to β2GPI and facilitates presentation of the cryptic epitopes of β2GPI as a consequence of antigen processing. In addition, some of our group (E.M. and K.K.) reported that stable and nondissociable β2GPI-oxLDL complexes were frequently detected in sera from patients with APS and/or systemic lupus erythematosus, but not in healthy individuals.29 In addition, β2GPI is known to have antiatherosclerosis activity by preventing oxLDL uptake by macrophages via scavenger receptor, but binding of IgG anti-β2GPI antibodies to β2GPI-oxLDL complexes mediates atherosclerosis by promoting phagocytosis of macrophages via Fcγ receptor.29,–31 Furthermore, elevated levels of procoagulant microparticles were detected in patients with APS in association with anti-β2GPI antibodies and lupus anticoagulant.32,–34 The presence of a large quantity of anionic substances in circulation in patients with APS supports our proposed model.
Based on our model, therapeutic strategies that inhibit pathogenic anti-β2GPI antibody production should target interrupting the continuous autoimmune loop carried out by macrophages and β2GPI-reactive CD4+ T cells and B cells. These immune cells are already targets of therapies under consideration, such as the anti-CD20 chimeric antibody rituximab.35 Another potential therapeutic approach includes the removal of immune complexes consisting of β2GPI, anionic substance, and anti-β2GPI antibodies. Accordingly, plasma exchange and double filtration plasmapheresis, which theoretically remove such immune complexes, are shown to be effective for patients with intractable APS, including catastrophic APS.36,37 Alternatively, small molecules that inhibit Fc receptor downstream signaling would have beneficial effects in patients with APS by suppressing the generation of β2GPI cryptic peptides.38
In summary, excessive exposure to anionic surfaces may play a key role in maintaining the pathogenic anti-β2GPI antibody response in patients with APS. Further studies should focus on mechanisms that prime the autoimmune loop and development of novel therapeutic strategies targeting the pathogenic process.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Yuka Okazaki and Takahide Arai for expert technical assistance.
This work was supported by a grant from the Japanese Ministry of Health, Welfare, and Labor, and a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture.
Authorship
Contribution: Y.Y., N.S., J.K., K.K., and E.M. performed experiments; Y.Y. and M.K. analyzed results and made the figures; Y.Y. and M.K. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Masataka Kuwana, Division of Rheumatology, Department of Internal Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan; e-mail:kuwanam@sc.itc.keio.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal