Activation-induced cell death (AICD) is involved in peripheral tolerance by controlling the expansion of repeatedly stimulated T cells via an apoptotic Fas (CD95; APO-1)–dependent pathway. The TNFRSF-6 gene encoding Fas is mutated in children suffering from autoimmune lymphoproliferative syndrome (ALPS), which is characterized by lymphoproliferation and autoimmunity. We examined AICD in Fas-deficient T cells from ALPS patients. We showed that primary activated Fas-deficient T cells die by apoptosis after repeated T cell antigen receptor (TCR) stimulation despite resistance to Fas-mediated cell death. This Fas-independent AICD was found to be mediated through a cytotoxic granules-dependent pathway. Cytotoxic granules-mediated AICD was also detected in normal T lymphocytes though to a lesser extent. As expected, the cytotoxic granules-dependent AICD was abolished in T cells from Rab27a- or perforin-deficient patients who exhibited defective granules-dependent cytotoxicity. Supporting an in vivo relevance of the cytotoxic granules-dependent AICD in ALPS patients, we detected an increased number of circulating T lymphocytes expressing granzymes A and B. Altogether, these data indicated that the cytotoxic granules-dependent cell death in ALPS may compensate for Fas deficiency in T lymphocytes. Furthermore, they identified a novel AICD pathway as a unique alternative to Fas apoptosis in human peripheral T lymphocytes.
Introduction
To ensure a proper immune response to foreign antigen while avoiding self-damaging responses, the size of the lymphocyte pool must be stringently regulated.1 In the case of T lymphocytes, 2 major mechanisms control the termination of the immune response. The activation cell-autonomous death (ACAD) ensures the elimination of cells that have responded to foreign antigen via an intrinsic apoptotic signaling.2 The activation-induced cell death (AICD) eliminates repeatedly stimulated T lymphocytes by an extrinsic apoptotic signaling.3 AICD is mediated through the interaction between the death receptor (DR) Fas and its ligand FasL expressed on activated T cells. Oligomerization of Fas leads to the formation of the death-inducing signaling complex (DISC) that initiates apoptosis.4 The role of the Fas molecule in controlling T-lymphocyte homeostasis has been initially underscored in natural mutant mice for Fas (Lpr and Lprcg) and FasL (gld) genes.5,6 Given that (1) central tolerance is overall normal in these animals, (2) lymphoproliferation occurs even in a germ-free environment and is frequently associated with autoimmunity, and (3) Fas limits the homeostatic proliferation of peripheral T cells,7 Fas probably controls the deleterious activation of self-reactive T cells.8
In humans, mutations in TNFRSF-6 gene encoding Fas are associated with the autoimmune lymphoproliferative syndrome (ALPS), a disorder in which defects in apoptosis lead to the accumulation of nonmalignant lymphocytes and autoimmunity. Most patients suffer an early onset of lymphadenopathy and (or) splenomegaly, an expansion of a normally rare population of CD4−/CD8−/T-cell antigen receptor (TCR)αβ+ double-negative (DN) T cells.9,10 Besides a few cases of homozygous mutations (ALPS-0),11,12 patients mainly carry heterozygous germ line (ALPS-Ia)13,14 or somatic (ALPS-Im) dominant Fas mutations.15 Other genetic defects affecting Fas signaling pathway might account for ALPS. A homozygous FasL mutation was reported in one case16 and 2 caspase-10 mutations were identified in 2 other siblings17 presenting with the syndrome. Of note, a human caspase-8 deficiency identified in consanguineous siblings led to combined immunodeficiency and pleiotropic proliferative defects.18 This observation highlights the role of caspase-8 not only in the Fas pathway but also in the antigen receptor–induced proliferation.
The mechanisms of AICD have been well documented in mice.2,19 However, less is known concerning human AICD. To explore the implication of Fas-independent apoptosis in human AICD, we used Fas-deficient T cells from ALPS patients to study AICD in the absence of functional Fas signaling. In this attempt, we set up an in vitro AICD assay based on repeated antigenic stimulation. We found that a perforin-dependent AICD occurs in Fas-deficient T cells. Because ALPS T cells were found to overexpress lytic-granule content in the periphery, these findings support the concept that the Fas/FasL and the lytic-granule pathways are interconnected and that both participate in the control of T-lymphocyte AICD in humans.
Patients, materials, and methods
Patients
Twenty-two patients were enrolled in the study. Patients presented with ALPS characterized by a lymphoproliferative syndrome consisting in splenomegaly and/or lymphadenopathy and/or autoimmune manifestations. Three ALPS-0 patients carried homozygous Fas mutation leading to complete functional Fas defects.11 For the ALPS-I patients, phenotypic, genetic, and functional analysis showed an elevated proportion of the TCRαβ+/CD4−/CD8− DN lymphocytes (range, 4%-55%), a heterozygous mutation in the Fas gene, and a defect in anti-CD95 monoclonal antibody-induced apoptosis, as previously described for such patients.7 The AICD experiments have been conducted on T cells from 9 patients (P1 to P9), 2 relatives (P1's father and P4's father), and 8 age-matched controls (CT). One patient with familial lymphohistiocytosis type-2 (FLH) carrying a homozygous Perforin missense mutation (C1376T) leading to impaired cytotoxicity20 was also enrolled, as well as one with the Griscelli syndrome (GS), carrying 2 heterozygous RAB27A missense mutations (T209C and C227T) affecting effector binding activity (M.M. and G.d.S.-B., unpublished data, August 2006). All patients, or their parents or guardians were provided written informed consent, validated by the Ethics Committee (Comité Consultatif pour la Protection des Personnes en Recherche Biomédicale) from the Necker Hospital, in accordance with the Declaration of Helsinki.
Isolation and fractionation of lymphocytes
Mononuclear cells were isolated from heparinized blood samples (peripheral blood mononuclear cells [PBMC]) or from spleen-cell suspension (SCS) (in the case of P3) by density gradient centrifugation (Lymphoprep; Abcys, Paris, France).
Fas and activation-induced cell death sensitivity
PBMC or SCS (106/mL) were activated either by 100 ng/mL of soluble mitogenic anti-CD3 monoclonal antibody (mAb) (OKT3 clone) or by 1 μg/mL of toxic-shock syndrome toxin-1 (TSST-1; Toxin Technology, Madison, WI) in Panserin medium (Dutscher, Brumath, France) completed with 5% of heat-inactivated pooled human serum from rhesus AB donors (EFS, Rungis, France) and 2 mM of glutamine. At day 3, dead cells were removed by density gradient centrifugation. T cells were maintained in 100 U/mL of human recombinant interleukin (IL)–2 until day 9. Fresh medium was replaced at days 5 and 8 of culture. In some experiments, CD8+ lymphocytes were magnetically depleted by positive selection using CD8-phycoerythrin (PE) and anti-PE magnetic beads (Miltenyi Biotech, Auburn, CA) before stimulation. For induction of AICD, 3 × 105 activated T cells were restimulated in the presence of 100 U/mL of IL-2 for 18 hours, with either 1 μg/mL of plated-bound anti-CD3 (OKT3 clone) or with 1 μg/mL of soluble TSST-1, in 96-well flat-bottom plates. Fas sensitivity was tested using cross-linked anti-CD95 mAb APO1.3 as previously described.14 In some experiments, AICD was compared with Fas-induced apoptosis after 6 hours of stimulation.
Conjugate formation and fluorescence staining
Day 9 TSST-1 activated T cells (106) were restimulated with TSST-1 for 10 minutes at 37°C, plated on glass cover slips coated with poly-l-lysine (Sigma, St. Louis, MO), and further incubated for 20 minutes at 37°C. Cover slips were placed on ice and cells were stained with anti-CD8-allophycocyanin (APC) mAb (BD Bioscience, San Jose, CA) and then fixed by incubation for 15 minutes in 3.7% (weight/volume) paraformaldehyde. Cells were then incubated for 45 minutes with antiperforin-fluorescein isothiocyanate (FITC) deltaG9 mAb in a permeabilizing buffer (phosphate-buffered saline [PBS], 1 mg/mL bovine serum albumin [BSA], and 0.05% (weight/volume) saponin; Sigma) and were washed. Cells were then incubated for 5 minutes with 0.3 μM DAPI (4,6-diamidino-2-phenylindole), were washed, and were mounted on slides in medium containing Mowiol antifading agent (Calbiochem, San Diego, CA). Samples were observed with an Axioplan 2 microscope (Zeiss, Jena, Germany). Eight-bit images were acquired with a Camera Spot (Diagnostic Instruments, Sterling Heights, MI) version 3.0 and a Zeiss 100×/1.3 oil IRIS objective. Detectors were set to detect an optimal signal below the saturation limits. Image sets to be compared were acquired with IP Lab software (Scanalytics; BD Bioscience) as grayscale pictures with the same acquisition settings and imported into Adobe Photoshop CS (Adobe, San Jose, CA) as TIFF files to be converted as green, red, or blue images.
Flow cytometry
On 100 μL of freshly drawn heparinized peripheral blood, splenocytes or activated T cells were surface-stained with anti-TCRαβ, anti-TCRVβ2, anti-CD95 (Immunotech, Marseille, France), anti-CD3, anti-CD4, and anti-CD8 mAb (BD Bioscience) for 1 hour on ice. Erythrocytes were eliminated using fluorescence-activated cell sorter (FACS)-lysing solution (BD Bioscience) as indicated by the manufacturer. For intracellular staining, cells were made permeable with Perm-2 solution according to the manufacturer's instructions (BD Bioscience) and labeled with the following antibodies: antigranzyme-A (R&D, Minneapolis, MN), antigranzyme-B (Hölzel Diagnostika, Cologne, Germany), and antiperforin (Ancell, Bayport, MN). Cells were analyzed on FACS-Calibur instrument using CellQuest software (BD Bioscience).
Apoptosis detection
Cell death was determined as the percentage of hypodiploid-nuclei (HN) assessed by propidium iodide staining, as described previously.14 Apoptosis was also determined by evaluating phosphatidylserine (PS) exposure in the outer leaflet of the cytoplasmic membrane with APC-conjugated Annexin-V (Ancell) in combination with 7-amino-actinomycin D (7-AAD) exclusion dye (R&D) 6 hours after reactivation. The percentage of induced apoptosis was calculated as 100 × [percentage of experimental HN − percentage of spontaneous HN]/[100 − % of spontaneous HN].
Statistical analysis
The unpaired Student t test was performed using Prism software (GraphPad Software, San Diego, CA). ns indicates not significant; 2-tailed *P value less than .05; **P value less than .005; and ***P value less than .001.
Results
Superantigen activation-induced cell death of T lymphocytes
Repeated stimulations of T lymphocytes via their antigen-receptor (TCR) induce activation-induced cell death (AICD) through a Fas–FasL interaction.21,22 To mimic repeated antigen-stimulation–induced cell death in vitro, we tested the sensitivity to AICD of T cells activated by the toxic shock syndrome toxin-1 (TSST-1) compared with a polyclonal mitogenic anti-CD3 stimulation. TSST-1 specifically binds to T-cell receptor Vβ2 (TCR-Vβ2)–positive T cells.23 This TCR subfamily is used by 5% to 9% of peripheral T cells (van den Beemd et al24 and data not shown). After 9 days of culture, both anti-CD3– and TSST-1–activated PBMC consisted of more than 90% CD3+/CD95+ T cells. Although TSST-1–activated T-cell blasts expressed either CD4 or CD825 at a comparable ratio to that found in CD3-activated T cells (see legends to Figures 1 and 3), they comprised around 85% of Vβ2+ lymphocytes (Figure 1 and data not shown). In contrast, the proportions of Vβ2+ lymphocytes remained unchanged after anti-CD3 mAb activation (Figure 1A and data not shown). TSST-1 or anti-CD3 mAb-stimulated T cells displayed a similar sensitivity toward Fas cross-linking or anti-CD3 mAb restimulation-induced cell death, indicating that T cells were optimally activated in both situations (Figure 1B). However, the level of cell death induced by TSST-1 restimulation was significantly higher in TSST-1 cultures than in CD3 cultures (Figure 1B). TSST-1-induced cell death was found to be proportional to the percentage of activated Vβ2+ T cells at the time of superantigen restimulation (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Thus, these findings indicate that the expansion/reactivation by TSST-1 represents a suitable assay for studying AICD.
TSST-1-induced AICD. T cells from healthy controls were activated with either anti-CD3 or TSST-1 as described in “Fas and activation-induced cell death sensitivity.” (A) Day 9 activated lymphocytes were double stained for CD3 and TCR-Vβ2 expression or for CD95 expression (gray histograms). One representative experiment of 5 is shown. The proportion of CD4+ T cells is not significantly different in anti-CD3-activated (61.4% ± 19.1%) vs TSST-1-activated (58.5% ± 11.9%) blasts (data not shown). Numbers on the plots are percentages of total cells. (B) T cells were reactivated (2°) at day 9 after initial stimulation (1°) by plate-bound anti-CD3 (░) or soluble TSST-1 (■) for 18 hours. At the same time, sensitivity to Fas-induced apoptosis was assessed using cross-linked agonistic anti-CD95 (APO1.3) mAb (□). Apoptosis was determined as described in “Apoptosis detection.” Values are the means (± [SEM]) of duplicated determinations of 5 independent experiments with 5 unrelated donors. ns indicates not significant; **P < .005; and ***P < .001.
TSST-1-induced AICD. T cells from healthy controls were activated with either anti-CD3 or TSST-1 as described in “Fas and activation-induced cell death sensitivity.” (A) Day 9 activated lymphocytes were double stained for CD3 and TCR-Vβ2 expression or for CD95 expression (gray histograms). One representative experiment of 5 is shown. The proportion of CD4+ T cells is not significantly different in anti-CD3-activated (61.4% ± 19.1%) vs TSST-1-activated (58.5% ± 11.9%) blasts (data not shown). Numbers on the plots are percentages of total cells. (B) T cells were reactivated (2°) at day 9 after initial stimulation (1°) by plate-bound anti-CD3 (░) or soluble TSST-1 (■) for 18 hours. At the same time, sensitivity to Fas-induced apoptosis was assessed using cross-linked agonistic anti-CD95 (APO1.3) mAb (□). Apoptosis was determined as described in “Apoptosis detection.” Values are the means (± [SEM]) of duplicated determinations of 5 independent experiments with 5 unrelated donors. ns indicates not significant; **P < .005; and ***P < .001.
AICD of Fas-defective T lymphocytes
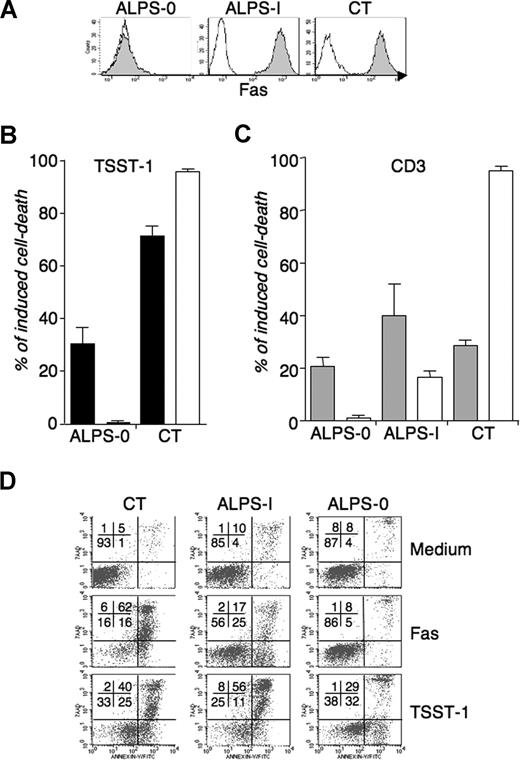
We next compared the intensity of Fas-mediated apoptosis induced by agonistic anti-CD95 mAb to activation-induced cell death triggered by TSST-1 restimulation of TSST-1-activated T cells from Fas-defective patients, their healthy mutated relatives, or healthy controls. The percentages of CD3+/TCR-Vβ2+ lymphocytes and the CD4/CD8 ratio in ALPS samples were overall similar to those detected in control donors both in PBMC and in day 9 cultured cells (data not shown). As expected, lymphocytes from ALPS-Ia patients displayed defective in vitro Fas-induced apoptosis compared with controls (Figure 2A). In contrast, TSST-1 restimulation induced a robust cell death of all ALPS lymphocytes, proportional to the percentage of activated Vβ2+ T cells (Figure 2B and data not shown). In addition, intensity of AICD did not correlate to the magnitude of Fas-induced apoptosis. For instance, lymphocytes from P1, fP1, and P3, which exhibited profound Fas-induced apoptosis deficiency, displayed very high levels of TSST-1-triggered AICD (Figure 2). To exclude a role of the residual Fas-dependent apoptosis in ALPS-Ia lymphocytes, we measured AICD in the absence of functional Fas using T cells from 2 unrelated patients with a complete Fas deficiency (ALPS-0). The patients had homozygous mutations in the Fas gene, resulting in a complete Fas expression deficiency (Figure 3A). CD3- or TSST-1-activated T-cell blasts from this patient were fully resistant to Fas-induced apoptosis (Figure 3B,C), as previously reported using phytohemagglutinin (PHA) stimulation (Kischkel et al4 and data not shown). TSST-1-activated cell death was readily detectable in spite of the absence of Fas expression, although with a reduced magnitude compared with controls and ALPS-Ia. Similar results were obtained with CD3-activated T cells restimulated with anti-CD3 mAb, confirming that the observed Fas-independent cell death is triggered upon TCR restimulation (Figure 3C). To determine whether Fas-independent T-cell death induced by TSST-1 reactivation in Fas-defective patients is apoptotic, phosphatidylserine (PS) exposure was evaluated using Annexin-V staining. PS+ cells were detected after restimulation with TSST-1 in T cells from the ALPS-0 (P7) patient, confirming that cells died by a mechanism of apoptosis (Figure 3D). Similarly, PS exposure was also observed in cells from ALPS-I (P3) and CT, as it was detected during Fas-induced apoptosis. These results indicate that in a context of Fas deficiency, an alternative mechanism of apoptosis could regulate T-cell fate after repeated TCR-mediated stimulation.
AICD in lymphocytes from Fas-defective patients and healthy controls. TSST-1 AICD (B) was compared with Fas sensitivity (A) of T cells from 6 ALPS-I patients (P1 to P6), from 2 healthy mutation positive relatives (fP1 and fP6), and from eight age-related control donors. Experiments were performed as described in Figure 1 using TSST-1/TSST-1 activation/restimulation. The shaded gray zones indicate means of apoptosis induced by Fas or TSST-1 AICD in control-cells (n = 21; in panel A, mean = 89% ± 2% [SEM]; in panel B, mean = 68% ± 2%).
AICD in lymphocytes from Fas-defective patients and healthy controls. TSST-1 AICD (B) was compared with Fas sensitivity (A) of T cells from 6 ALPS-I patients (P1 to P6), from 2 healthy mutation positive relatives (fP1 and fP6), and from eight age-related control donors. Experiments were performed as described in Figure 1 using TSST-1/TSST-1 activation/restimulation. The shaded gray zones indicate means of apoptosis induced by Fas or TSST-1 AICD in control-cells (n = 21; in panel A, mean = 89% ± 2% [SEM]; in panel B, mean = 68% ± 2%).
AICD of lymphocytes with a Fas deficiency. AICD was compared with Fas sensitivity in one Fas-deficient ALPS-0 patient and one highly Fas-defective ALPS-I patient (P3). (A) CD95 expression (gray histograms) on day 9 TSST-1-activated T cells from one ALPS-0 patient (P7), one ALPS-I patient (P3), and control (one representative evaluation of 3). (B) TSST-1-activated T cells were restimulated with TSST-1 ( ) or (C) CD3-activated T cells were restimulated with anti-CD3 (
) or (C) CD3-activated T cells were restimulated with anti-CD3 ( ), and Fas-induced apoptosis was measured in T cells from each culture using agonistic anti-CD95 (
), and Fas-induced apoptosis was measured in T cells from each culture using agonistic anti-CD95 ( ). Means and SEM of are from 5 (B) and 3 (C) independent experiments. As for WT lymphocytes, the proportion of CD4+ T cells is not significantly different in anti-CD3-activated (53.2% ± 11.8%) versus TSST-1-activated (57.7% ± 13.8%) blasts (data not shown). (D) Apoptotic cell death was detected after 6 hours of stimulation of day 9 TSST-1-activated T cells from ALPS-0, ALPS-I, and healthy control donor (CT) after reactivation by soluble TSST-1 or CD95 cross-linking, using Annexin-V detection (x axis) and 7AAD exclusion dye (y axis). One representative experiment of 3. Numbers on plots are percentages of total cells.
). Means and SEM of are from 5 (B) and 3 (C) independent experiments. As for WT lymphocytes, the proportion of CD4+ T cells is not significantly different in anti-CD3-activated (53.2% ± 11.8%) versus TSST-1-activated (57.7% ± 13.8%) blasts (data not shown). (D) Apoptotic cell death was detected after 6 hours of stimulation of day 9 TSST-1-activated T cells from ALPS-0, ALPS-I, and healthy control donor (CT) after reactivation by soluble TSST-1 or CD95 cross-linking, using Annexin-V detection (x axis) and 7AAD exclusion dye (y axis). One representative experiment of 3. Numbers on plots are percentages of total cells.
AICD of lymphocytes with a Fas deficiency. AICD was compared with Fas sensitivity in one Fas-deficient ALPS-0 patient and one highly Fas-defective ALPS-I patient (P3). (A) CD95 expression (gray histograms) on day 9 TSST-1-activated T cells from one ALPS-0 patient (P7), one ALPS-I patient (P3), and control (one representative evaluation of 3). (B) TSST-1-activated T cells were restimulated with TSST-1 ( ) or (C) CD3-activated T cells were restimulated with anti-CD3 (
) or (C) CD3-activated T cells were restimulated with anti-CD3 ( ), and Fas-induced apoptosis was measured in T cells from each culture using agonistic anti-CD95 (
), and Fas-induced apoptosis was measured in T cells from each culture using agonistic anti-CD95 ( ). Means and SEM of are from 5 (B) and 3 (C) independent experiments. As for WT lymphocytes, the proportion of CD4+ T cells is not significantly different in anti-CD3-activated (53.2% ± 11.8%) versus TSST-1-activated (57.7% ± 13.8%) blasts (data not shown). (D) Apoptotic cell death was detected after 6 hours of stimulation of day 9 TSST-1-activated T cells from ALPS-0, ALPS-I, and healthy control donor (CT) after reactivation by soluble TSST-1 or CD95 cross-linking, using Annexin-V detection (x axis) and 7AAD exclusion dye (y axis). One representative experiment of 3. Numbers on plots are percentages of total cells.
). Means and SEM of are from 5 (B) and 3 (C) independent experiments. As for WT lymphocytes, the proportion of CD4+ T cells is not significantly different in anti-CD3-activated (53.2% ± 11.8%) versus TSST-1-activated (57.7% ± 13.8%) blasts (data not shown). (D) Apoptotic cell death was detected after 6 hours of stimulation of day 9 TSST-1-activated T cells from ALPS-0, ALPS-I, and healthy control donor (CT) after reactivation by soluble TSST-1 or CD95 cross-linking, using Annexin-V detection (x axis) and 7AAD exclusion dye (y axis). One representative experiment of 3. Numbers on plots are percentages of total cells.
Cytotoxic granule-dependent AICD
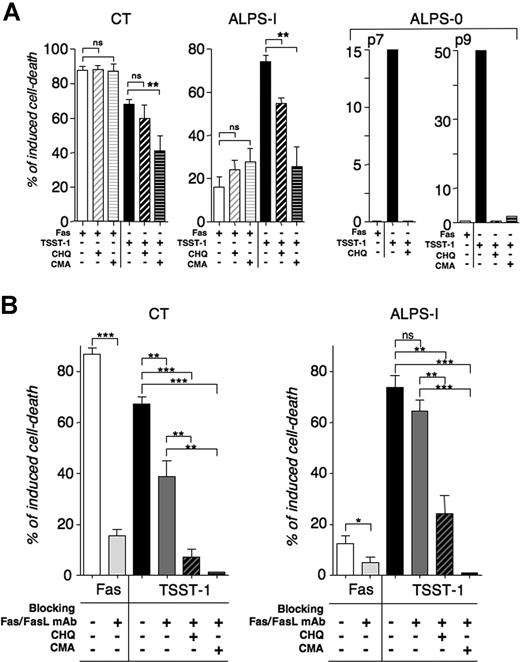
It has been proposed that AICD could be mediated by a perforin-dependent pathway in murine lymphocytes.26 The proteolytic maturation of perforin under acidic conditions is required for its activity.27 The acidophilic compounds concanamycin-A (CMA) and chloroquine (CHQ) were shown to prevent its processing and thus cytotoxic activity.28,29 To test whether Fas-independent AICD observed in ALPS patients could be perforin dependent, we examined the effect of CMA and CHQ on TSST-1-induced cell death of Fas-deficient lymphocytes from ALPS-0 and ALPS-I patients (Figure 4A). Pretreatment of day 9 activated lymphocytes with CHQ or CMA totally abrogated restimulation-induced cell death in ALPS-0 (Figure 4A). Similarly, CHQ or CMA treatment significantly reduced the TSST-1-induced cell death in ALPS-Ia. Of note, the magnitude of the remaining AICD was similar to the magnitude of Fas-induced cell death. Therefore, the residual apoptosis after TSST-1 stimulation in CHQ- or CMA-treated cells can most probably be attributed to the residual Fas-mediated activity in these cells. A mild but significant inhibitory effect of CMA was also observed in AICD of control lymphocytes. Because neither CMA nor CHQ treatment affected apoptotic Fas signaling, these results strongly support the notion that cytotoxic granules can mediate Fas-independent AICD. It thus appeared that 2 mechanisms of AICD were concomitantly active after TSST-1 activation. Besides Fas, other members of the tumor necrosis factor R (TNF-R) family have been reported to induce AICD in activated human T lymphocytes.30 To assess the contribution of additional apoptotic pathway(s), we analyzed the TSST-1 AICD in the presence of blocking anti-Fas and anti-FasL mAbs, in combination with CHQ or CMA treatment. As expected, blocking Fas impeded apoptosis induced by agonistic anti-Fas mAb (APO.1.3) in lymphocytes from CT and ALPS-Ia and prevented restimulation-induced cell death of lymphocytes expressing wild-type (WT) Fas (CT, Figure 4B). However, blocking Fas had no detectable consequence on TSST-1 AICD of mutated lymphocytes (Figure 4B). By combining inhibitors of Fas/FasL with CMA, a full inhibition of TSST-1-induced cell death in CT and ALPS-I was observed (Figure 4B), indicating that there was no alternative acidification-independent apoptosis mechanism involved in the TSST-1-induced AICD. Conversely, the TSST-1-induced AICD of T cells from patients deficient in the cytotoxic-granule pathway machinery was tested. Addition of CHQ on AICD from Rab-27a and perforin-deficient T cells was analyzed in comparison to ALPS-I and CT T cells. CHQ partially inhibited AICD in CT and ALPS-I, whereas it had no effect on AICD from Rab-27a and perforin-deficient lymphocytes (Figure 5A). Fas-induced apoptosis was also unaffected by CHQ in these cells (Figure 5B and data not shown). Granule-dependent cytotoxicity is characterized by the rapid polarization of the lytic machinery toward the region of contact between the cytotoxic and the target cell, the secretory synapse.31,32 This is accompanied by the formation of conjugate between effector and target cells. We therefore performed analysis of conjugate formation during TSST-1-induced cell death. We indeed similarly observed polarization of perforin-containing granules and conjugate formation during TSST-1 restimulation in lymphocytes from ALPS patients and controls (Figure 5B). Interestingly, both CD8 and CD4 T cells polarized their granules toward neighboring CD8 or CD4 T lymphocytes. This is consistent with the fact that CHQ-sensitive AICD was detected in CD8-depleted cultures from P3 (data not shown). These results demonstrate that AICD induced by the TSST-1/TSST-1 expansion/restimulation system results from both the Fas–FasL and the cytotoxic-granule pathways-mediated apoptosis. The granule-dependent cell death could thus partially compensate for a Fas deficiency in in vitro TSST-1-induced cell death.
Cooperative inhibition of AICD of control and ALPS lymphocytes by blocking cytotoxic-granule and Fas-mediated cell death. (A) The effect of the acidophilic compounds chloroquine (CHQ) and concanamycin-A (CMA) was tested on Fas- and TSST-1-induced cell death on TSST-1 T-cell blasts. Thirty minutes before the test, activated lymphocytes were incubated at 37°C with 0.1 mM CHQ or 10 nM CMA. Treated cells were then submitted to AICD or Fas-induced apoptosis as described previously. Data are means (± SEM) of duplicates from independent experiments (CT: n = 6; ALPS-I (P3): n = 3; ALPS-0 (P7 and P8) was tested once). (B) Effect of Fas/FasL blocking mAb was tested on AICD by incubating T cells with 10 μg/mL of antagonistic anti-Fas (SM1/23) and anti-FasL (2C10) mAb 30 minutes before reactivation ( ). These antibodies efficiently inhibit Fas-induced cell death by agonistic anti-CD95 (APO1.3) mAb (
). These antibodies efficiently inhibit Fas-induced cell death by agonistic anti-CD95 (APO1.3) mAb ( ). ns indicates not significant; *P < .05; **P < .005; ***P < .001.
). ns indicates not significant; *P < .05; **P < .005; ***P < .001.
Cooperative inhibition of AICD of control and ALPS lymphocytes by blocking cytotoxic-granule and Fas-mediated cell death. (A) The effect of the acidophilic compounds chloroquine (CHQ) and concanamycin-A (CMA) was tested on Fas- and TSST-1-induced cell death on TSST-1 T-cell blasts. Thirty minutes before the test, activated lymphocytes were incubated at 37°C with 0.1 mM CHQ or 10 nM CMA. Treated cells were then submitted to AICD or Fas-induced apoptosis as described previously. Data are means (± SEM) of duplicates from independent experiments (CT: n = 6; ALPS-I (P3): n = 3; ALPS-0 (P7 and P8) was tested once). (B) Effect of Fas/FasL blocking mAb was tested on AICD by incubating T cells with 10 μg/mL of antagonistic anti-Fas (SM1/23) and anti-FasL (2C10) mAb 30 minutes before reactivation ( ). These antibodies efficiently inhibit Fas-induced cell death by agonistic anti-CD95 (APO1.3) mAb (
). These antibodies efficiently inhibit Fas-induced cell death by agonistic anti-CD95 (APO1.3) mAb ( ). ns indicates not significant; *P < .05; **P < .005; ***P < .001.
). ns indicates not significant; *P < .05; **P < .005; ***P < .001.
AICD of lymphocytes with cytotoxic-granule deficiencies. (A) CHQ sensitivity of AICD of lymphocytes from Rab-27a (GS)– and perforin (FLH)–deficient patients was compared with those of ALPS-I and CT. TSST-1 T-cell blasts were restimulated with soluble TSST-1 (■) during 6 hours in the presence of CHQ (▨), compared with Fas-induced cell death (□). (n = 2 for CT and GS; P3 and FLH were tested once). (B) Day 9 TSST-1 T-cell blasts were stimulated 30 minutes with soluble TSST-1, and then conjugates were analyzed. Cytotoxic granules were visualized by FITC-labeled anti-perforin deltaG9 mAb (green). Nuclei were labeled with DAPI dye (blue) and CD8+ cells were stained with anti-CD8 APC (red). CD4+ T cells were left unlabeled. Representative images from 2 independent experiments are shown. Scale bars represent 5 μm. In all conditions except FLH, all observed conjugates are formed with at least one cell polarizing perforin-containing granules. The number of conjugates observed is not significantly different in the 5 conditions tested (mean = 49% ± 5% [SEM]).
AICD of lymphocytes with cytotoxic-granule deficiencies. (A) CHQ sensitivity of AICD of lymphocytes from Rab-27a (GS)– and perforin (FLH)–deficient patients was compared with those of ALPS-I and CT. TSST-1 T-cell blasts were restimulated with soluble TSST-1 (■) during 6 hours in the presence of CHQ (▨), compared with Fas-induced cell death (□). (n = 2 for CT and GS; P3 and FLH were tested once). (B) Day 9 TSST-1 T-cell blasts were stimulated 30 minutes with soluble TSST-1, and then conjugates were analyzed. Cytotoxic granules were visualized by FITC-labeled anti-perforin deltaG9 mAb (green). Nuclei were labeled with DAPI dye (blue) and CD8+ cells were stained with anti-CD8 APC (red). CD4+ T cells were left unlabeled. Representative images from 2 independent experiments are shown. Scale bars represent 5 μm. In all conditions except FLH, all observed conjugates are formed with at least one cell polarizing perforin-containing granules. The number of conjugates observed is not significantly different in the 5 conditions tested (mean = 49% ± 5% [SEM]).
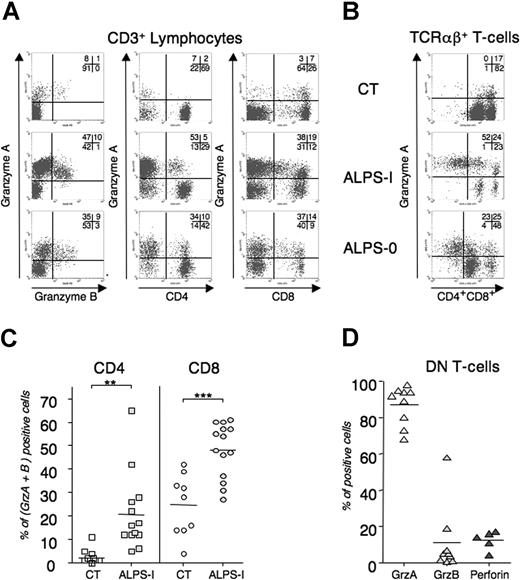
Granule content of peripheral T lymphocytes in controls and ALPS
Because of the role of cytolytic granules mediating AICD of Fas-deficient T cells, we examined the lytic machinery in ALPS lymphocytes. Granzymes consist in a family of granule-specific serine proteases that rapidly trigger target cell death.33 Granzyme-A (GrzA) and granzyme-B (GrzB) are the most abundant and the best characterized executioners of granule-mediated cytotoxicity. To quantify the granule content of peripheral T cells in ALPS patients, we performed flow cytometry analysis using GrzA and GrzB mAb. As depicted in Figure 6A, ALPS-I CD3+ splenocytes and ALPS-0 peripheral-blood T lymphocytes expressed large amounts of both granzymes, compared with blood lymphocytes from an age-matched healthy control donor. Interestingly, both CD8 and CD4 T cells expressed granzymes, whereas they were only faintly detected in WT CD4 lymphocytes (CT). We next extended the flow-cytometry analysis to blood samples from 13 unrelated APLS-I patients compared with 9 age-matched healthy control donors. CD8+ T cells from ALPS-I patients significantly overexpressed GrzA and GrzB, compared with CT. Again, granzymes were detected in CD4+ ALPS-Ia lymphocytes, thus confirming the in vitro findings. Overall, these results indicate that Fas-deficient conditions lead to overrepresentation of lytic granule machinery in human T lymphocytes. Finally, the granule content of TCRαβ+/CD4−/CD8− DN T cells that specifically accumulated in ALPS was analyzed. As depicted in Figure 6, ex vivo DN T cells from ALPS-I and ALPS-0 were mostly GrzA positive. Similarly, GrzA is detected in all DN T cells from all ALPS-Ia tested (mean = 85%, n = 9) whereas GrzB and perforin are not detected.
Granule content of peripheral T lymphocytes in control and ALPS. Splenocytes (ALPS-I in panels A and B) or freshly drawn heparinized peripheral blood samples were stained for TCRαβ, CD3, CD4, and CD8 expression. Cells were next stained for GrzA, GrzB, and perforin content. (A) Intracellular GrzA and -B content in total CD3+ (left), in CD3+/CD4+ (middle), or in CD3+/CD8+ (right) and (B) GrzA content in DN T cells (TCRαβ+ CD4−CD8−) are represented for P3 (ALPS-I), P8 (ALPS-0), and CT. Numbers on plots are percentages of total cells. (C) The percentage of GrzA+ and GrzB+ in CD4+ or CD8+ T cells from 13 ALPS-I patients and 9 aged-related healthy donors or (D) GrzA, GrzB, and perforin expression in DN T cells from 5 to 9 ALPS-I. Percentages (dots) and means (bars) are plotted. **P < .005; ***P < .001
Granule content of peripheral T lymphocytes in control and ALPS. Splenocytes (ALPS-I in panels A and B) or freshly drawn heparinized peripheral blood samples were stained for TCRαβ, CD3, CD4, and CD8 expression. Cells were next stained for GrzA, GrzB, and perforin content. (A) Intracellular GrzA and -B content in total CD3+ (left), in CD3+/CD4+ (middle), or in CD3+/CD8+ (right) and (B) GrzA content in DN T cells (TCRαβ+ CD4−CD8−) are represented for P3 (ALPS-I), P8 (ALPS-0), and CT. Numbers on plots are percentages of total cells. (C) The percentage of GrzA+ and GrzB+ in CD4+ or CD8+ T cells from 13 ALPS-I patients and 9 aged-related healthy donors or (D) GrzA, GrzB, and perforin expression in DN T cells from 5 to 9 ALPS-I. Percentages (dots) and means (bars) are plotted. **P < .005; ***P < .001
Discussion
Activation-induced cell death is a key mechanism of the immune system homeostasis, particularly of T lymphocytes.30 The Fas/FasL pathway and the cytotoxic granule pathway have been described as essential regulators of peripheral T-cell homeostasis.22,34,–36 This is highlighted by both forms of lymphoproliferative disorder in patients expressing defective cytotoxic granule function or defective Fas receptor.9,37 In the former condition, patients suffer uncontrolled proliferation of CD8+ T cells accompanied by massive tissue infiltration, macrophage activation, and hemophagocytosis. In the latter condition, patients suffer an autoimmune lymphoproliferative syndrome (ALPS), characterized by a benign tumoral syndrome, accumulation of TCRαβ DN T cells, and hyperglobulinemia, notably hyper IgG and IgA. Autoimmune manifestations occur in two-thirds of the ALPS patients, mostly as autoimmune cytopenia. Therefore in addition to being the molecular weapons of the cytotoxic T cells,38 these 2 cytotoxic pathways control the size of the effector lymphocyte pool. Cytotoxic granule release is involved in the regulation of CD8+ T-cell pool proliferation after infections with intracellular pathogens, whereas the Fas/FasL appears responsible for the elimination of autoreactive T cells.39,40 However, the implications of other death receptors in AICD remain unclear. In mice, the TNF as well as the TNF receptor apoptosis inducing ligand (TRAIL) pathways have been described as potential actors of AICD.19,41,42 The relative extent to which these pathways contribute to AICD in human lymphocytes remains unclear. To evaluate the contribution of Fas-independent pathways in AICD, we set up an in vitro model of AICD and took advantage of Fas-defective T cells obtained from ALPS patients. Our experimental model was based on specific antigen restimulation of Vβ2+ activated T cells by the bacterial superantigen TSST-I.23 This activation could be achieved by virtue of this superantigen to directly bind a major histocompatibility complex (MHC) Class II molecule expressed on activated T cells and the Vβ2 CDR2 loop of the TCR, rendering possible T–T lymphocytes antigen-specific activation.43,44 In this setting, we observed that T cells from ALPS patients exhibited an AICD comparable with control T cells but remained resistant to an agonistic anti-Fas treatment. To exclude that the residual Fas activity observed in ALPS-I patients may account for AICD, we performed the same experiments using T cells completely deficient for Fas. ALPS-0 patients carry homozygous null mutations thus leading to a complete expression defect as well as to a complete functional defect.11 Using these Fas-null cells, we reproducibly observed a TSST-1 and anti-CD3 triggered AICD. This finding provides evidence for a Fas-independent TCR-dependent cell death pathway. Consistently, a combination of antagonistic anti-Fas and anti-FasL only partially inhibited a TSST-1-triggered AICD in control T cells, whereas the same combination fully inhibited an anti-Fas-induced apoptosis. TSST-1-triggered AICD of the Fas-deficient T cells was shown to be mediated by perforin-containing cytolytic granules. Concanamycin A, and to a lesser extent chloroquine, which prevent proteolytic maturation of perforin by alkalinization of the acidic compartments, block TSST-1-triggered AICD. It is highly unlikely that these compounds could exert an inhibition of other pathways involved in triggering apoptosis. Indeed, under these conditions, apoptosis mediated by Fas and TRAIL-R, death receptors that share essential down-stream apoptotic signaling molecules, were not inhibited (Figure 4A and data not shown). T-cell blasts were not significantly sensitive to TRAIL-induced cell death (data not shown), excluding its implication in the Fas-independent AICD. Because no residual AICD was observed once both Fas/FasL and perforin pathways were inhibited, it is highly unlikely that other death pathway(s) contribute to AICD, at least in this setting.
Observation of conjugate formation and polarization of perforin-containing granules toward the synapse upon TSST-1 stimulation are also consistent with the finding of a granule-mediated AICD. The overrepresentation of granzyme A and B in CD8+ as well as CD4+ T cells from ALPS could also support such a model. GrzB containing lymphocytes have been also detected in the liver of ALPS presenting with chronic viral hepatitis.45 Conversely, cells genetically deficient in perforin or in Rab27a, both defects leading to defective granule-dependent cytolytic activity, exhibited a reduced AICD triggered by TSST-1 compared with control. In addition, AICD of T cells defective for the cytotoxic granule pathway was not inhibited by chloroquine, in contrast to what is observed in ALPS-I lymphocytes. The present data are consistent with the recent description of a role of endogenous GrzB in the elimination of human T cells (Th2 cells) upon TCR signaling.46,47 Granule exocytosis seems not to be involved in this setting.47 In contrast, the impaired AICD we observed in Rab27a-deficient lymphocytes suggests that degranulation occurred in our experimental setting. Indeed, degranulation was confirmed during TSST-1-induced AICD using a GrzA release assay (Menasche et al48 and data not shown).
Collectively, these findings show that both the Fas/FasL and the cytolytic granule pathways can mediate AICD, and that in a setting of Fas deficiency, the latter pathway may to some extent compensate the Fas defect. These data are consistent with the observed increased severity of the phenotype of Fas−/−/perforin−/− mice.36,49,50 This could be either the consequence of the activation of T cells in ALPS or evidence for a crosstalk between perforin/granzyme pathway and the Fas pathway. Finding normal AICD together with a defective Fas-induced apoptosis in cells from healthy mutant-positive relatives, in the absence of detectable in vivo activated cells, is in favor of a feedback mechanism exerted by the Fas pathway. The potential compensation of the defective Fas-mediated apoptosis in ALPS mediated by the perforin-dependent pathway might explain the recent finding that mild variation (N252S) of perforin could be a contributing factor to ALPS,51,52 though the influence on perforin function is controversial.53,54 However, the fact that AICD is equivalently detected in symptomatic and asymptomatic Fas-mutated patients in the same pedigree suggests that potential variation in these pathways does not account for intrafamilial variability in the penetrance of ALPS.55
Another finding provided by our study is the phenotype of the accumulated double negative T cells found in ALPS, as far as cytolytic proteins are concerned. These cells strongly express granzyme A whereas granzyme B and perforin are barely detected, making these cells unable to exert a cytolytic granule-dependent activity. This strongly contrasts with the above-discussed expression of granzyme B and perforin found in CD8 as well as CD4 T cells of ALPS patients. Because these DN T cells are considered as anergic “end cells” derived from single positive T cells,15,56 it suggests that a repressor mechanism is induced that down-regulated expression of granzyme B and perforin while sparing granzyme A. One possible mechanism may involve the anti-inflammatory cytokine IL-10. This cytokine is highly produced by DN T cells57 and has been shown to repress granzyme B mRNA expression in cytotoxic lymphocytes.58 This repressor mechanism could be similar to the mechanism involved in the modulation of the CD4 or CD8 coreceptors.59 This work shows that the 2 major TCR-triggered apoptotic pathways may be interregulated, contributing to homeostatic equilibrium of T cells. Further analysis of genetic disorders may provide clues in understanding how these pathways crosstalk.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from INSERM, the Agence Nationale de la Recherche (ANR; grant no. 05-MRAR-017), the Association pour la Recherche contre le Cancer (ARC), la Ligue Nationale contre le Cancer, la Ligue Parisienne contre le Cancer, and the European STREP (6th FP, autorome). V.M. was supported by postdoctoral fellowship from European Molecular Biology Organization (EMBO). M.M. was supported by doctoral fellowship from the Ministère de l'Éducation Nationale, de la Recherche et de la Technologie.
Authorship
Contribution: V.M. designed and performed research, analyzed and interpreted data, and wrote the paper; M.M. performed and analyzed research; G.d.S.-B. provided vital clinical information and samples from FLH and Rab27a patients and interpreted data; M.-C.S. performed research; B.R., N.A., B.F., F.l.D., and C.P. provided clinical information and blood sample from ALPS patients; A.F. provided essential clinical information from patients, designed research, interpreted data, and wrote the paper; and F.R.-L. designed research, interpreted the data, wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frédéric Rieux-Laucat, INSERM U768/Hôpital Necker-Enfants Malades, 149 Rue de Sèvres, 75015 Paris, France; e-mail:rieux@necker.fr.

![Figure 1. TSST-1-induced AICD. T cells from healthy controls were activated with either anti-CD3 or TSST-1 as described in “Fas and activation-induced cell death sensitivity.” (A) Day 9 activated lymphocytes were double stained for CD3 and TCR-Vβ2 expression or for CD95 expression (gray histograms). One representative experiment of 5 is shown. The proportion of CD4+ T cells is not significantly different in anti-CD3-activated (61.4% ± 19.1%) vs TSST-1-activated (58.5% ± 11.9%) blasts (data not shown). Numbers on the plots are percentages of total cells. (B) T cells were reactivated (2°) at day 9 after initial stimulation (1°) by plate-bound anti-CD3 (░) or soluble TSST-1 (■) for 18 hours. At the same time, sensitivity to Fas-induced apoptosis was assessed using cross-linked agonistic anti-CD95 (APO1.3) mAb (□). Apoptosis was determined as described in “Apoptosis detection.” Values are the means (± [SEM]) of duplicated determinations of 5 independent experiments with 5 unrelated donors. ns indicates not significant; **P < .005; and ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-05-088286/3/m_zh80240710300001.jpeg?Expires=1769232834&Signature=JMX6uoeDMwKrzJQPZwXIyHh6jKgYxmpzWqmXZj0nOtWN8TvkoT5nNh82SXH2nIS4LlZhI5mF8GV5FFr5hb~k6BDryO~DrYgpelPoKeB2BsPNSL5TD-5L0rdZF4uIT71tKhGjJMX9p9B73JQskZ03OdDupgJ-OFRC~GCEeqJgIdD~NlaT1mxAQugGjUvr-WCzsXtqYDTcivr1XqWfqpyK1ZAiCKEzTLU~wOaRGq~v4P40dkSdZ4T~i56T6mPsoDj6NvdRqxD2eJ4emsNyKTxIIOKFtk5AQ1Dci3lTSBcSQoQlRpnoZ3yoQlzSvYpRwoeb5AzwbAM40QrmGwRuFQpyZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. AICD in lymphocytes from Fas-defective patients and healthy controls. TSST-1 AICD (B) was compared with Fas sensitivity (A) of T cells from 6 ALPS-I patients (P1 to P6), from 2 healthy mutation positive relatives (fP1 and fP6), and from eight age-related control donors. Experiments were performed as described in Figure 1 using TSST-1/TSST-1 activation/restimulation. The shaded gray zones indicate means of apoptosis induced by Fas or TSST-1 AICD in control-cells (n = 21; in panel A, mean = 89% ± 2% [SEM]; in panel B, mean = 68% ± 2%).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-05-088286/3/m_zh80240710300002.jpeg?Expires=1769232834&Signature=NygeF7wHH35VO6KHeLpQixtiLrTKGE1NdG~-jI2wjRxeTXXwlRTT2wpoNVpu-RiFHxn07vTpuquBjuFpVTJMkN3Nkr-SbwK9s~B3c30mm6CcCb7Lae-mx-62vqbEby8WyZGBZHIfWyjGRAi7U8UNisJp1ayJL2mp2Vfnz~pNtK-TvHY3UMqJfYpPBHpoXqLdmH30i~hoVsTRFNh8RXgTYNaJQPTNeclppFFmSoLe~XHIrwsVBRaIvA0HTdMNBgD~5bkOHb-2~83DbA9KLCMTIUSCUPv8baJMVziHCvCRFjm3ebmd6ZSCF2wVRP-UzH0awJhEiItbznfP-ORPO0Lbbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. AICD of lymphocytes with cytotoxic-granule deficiencies. (A) CHQ sensitivity of AICD of lymphocytes from Rab-27a (GS)– and perforin (FLH)–deficient patients was compared with those of ALPS-I and CT. TSST-1 T-cell blasts were restimulated with soluble TSST-1 (■) during 6 hours in the presence of CHQ (▨), compared with Fas-induced cell death (□). (n = 2 for CT and GS; P3 and FLH were tested once). (B) Day 9 TSST-1 T-cell blasts were stimulated 30 minutes with soluble TSST-1, and then conjugates were analyzed. Cytotoxic granules were visualized by FITC-labeled anti-perforin deltaG9 mAb (green). Nuclei were labeled with DAPI dye (blue) and CD8+ cells were stained with anti-CD8 APC (red). CD4+ T cells were left unlabeled. Representative images from 2 independent experiments are shown. Scale bars represent 5 μm. In all conditions except FLH, all observed conjugates are formed with at least one cell polarizing perforin-containing granules. The number of conjugates observed is not significantly different in the 5 conditions tested (mean = 49% ± 5% [SEM]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-05-088286/3/m_zh80240710300005.jpeg?Expires=1769232834&Signature=V8-6Bx2UGYfG82ltLZaUafjVw3H6KBv8C09AmGbCQDd9XIS2dPysD-~yRJ3vUbTWLGQYKA57WAfcoOSPN1vQCPC10MSZwKbZ0PtGZ-PbB1Zhx9dIqQogbULoeH-WiXSrnmbydLSyYz-9NCOh9WaPY9myua2Dnx7ZGalMEfVNt0ozLWVs0603srCsJVP~KFff5wV~MckIERHkuaEVLVLNqntNUUai-0uh-clZDUoE70DMhTi9UEZPvKsn91IOz1BEYS2Q7i8HQij3810KdoAAvfRFLDFKIQkd3K~4zoTP~1teLNYHYPa~4OjwLoU5AT03korC0M00a2OFuObAnCYT~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)