Platelets release insulin-like growth factor-1 (IGF-1) from α granules upon activation. We have investigated the regulation of IGF-1 in Gi-dependent pathways leading to Akt activation and the role of IGF-1 in platelet activation. IGF-1 alone failed to induce platelet aggregation, but IGF-1 potentiated 2-MeSADP–induced platelet aggregation in a concentration-dependent manner. IGF-1 triggered platelet aggregation in combination with selective P2Y1 receptor activation. IGF-1 also caused platelet aggregation without shape change when combined with selective Gz stimulation by epinephrine, suggesting the role of IGF-1 in platelet aggregation by supplementing Gi pathways. The potentiating effect of IGF-1 was not affected by intracellular calcium chelation. Importantly, IGF-1 was unable to potentiate platelet aggregation by the phosphatidylinositol 3-kinase (PI3-K) inhibitor wortmannin, suggesting a critical regulation by PI3-K. Moreover, the potentiating effect of IGF-1 was abolished by the presence of PI3-K p110α inhibitor PIK-75. Stimulation of platelets with IGF-1 resulted in phosphorylation of Akt, a downstream effector of PI3-K, which was completely inhibited by wortmannin. IGF-1-induced Akt phosphorylation was abolished by PIK-75 suggesting the contribution of PI3-K p110α for activation of Akt by IGF-1. These results demonstrate that IGF-1 plays a role in potentiating platelet aggregation by complementing Gi- but not Gq-signaling pathways via PI3-K p110α.
Introduction
At the site of vascular injury, platelets aggregate and release growth factors such as insulin-like growth factors (IGFs), platelet-derived growth factor, epidermal growth factor, endothelial cell growth factor, and transforming growth factor-β.1,,,–5 IGF-1 plays an important role in wound healing and platelets release IGF-1 from α granules upon activation.6 ADP can mediate α-granule release in washed human platelets.7 Receptors for IGF-1 have been demonstrated on platelet surface membranes.8 A previous study has reported that IGF-1 can potentiate ADP-, collagen-, and thrombin-induced platelet aggregation.9 Recent study has shown that P2Y12 receptor–mediated Akt activation requires IGF-1 receptor in C6 glioma cells.10 However, the functional role of IGF-1 and its signaling mechanisms in platelet activation are unclear
IGF-1 mediates the biological function by binding to its transmembrane receptor (IGF-1 receptor), and the biological actions of IGF-1 are modulated by a family of at least 6 IGF-binding proteins.11 IGF-1 receptor contains a 30-residue signal peptide rich in polar amino acids, followed by α and β subunits.12 IGF-1 receptor is similar to the insulin receptor in structural features and consists of an α2β2 configuration, where 2 αβ subunits are linked by secondary disulfide bonds. Binding of IGF-1 to its receptor induces receptor autophosphorylation of 3 tyrosine residues in the intracellular kinase domain of the β subunit and results in activation of the tyrosine kinase activity of the IGF-1 receptor. The activated IGF-1 receptor phosphorylates intracellular substrate insulin receptor substrate-1 (IRS-1) and SH2-containing protein Shc (src homology domain–containing protein). Phosphorylated IRS-1, in turn, binds to certain SH2 domain–containing proteins such as phosphatidylinositol 3-kinase (PI3-K),13 Grb-2, and Syp.12 Activated PI3-K mediates Akt activation, which contributes to the survival signal of IGF-1.14,15
ADP is an important agonist for platelet activation and causes platelets to change their shape, aggregate, and release contents of granules. ADP-induced platelet aggregation requires coactivation of the Gq-coupled P2Y1 and Gi-coupled P2Y12 receptors.16,17 The Gq-coupled P2Y1 receptor is responsible for inositol phosphate formation through the activation of phospholipase C, leading to mobilization of calcium and platelet shape change.18 The Gi-coupled P2Y12 receptor leads to the inhibition of adenylyl cyclase (AC)19,20 and activates PI3-K,21 Akt,22,23 and Rap1b.24 Moreover, Gz signaling triggered by epinephrine mimicked the P2Y12 receptor–mediated signaling events, thus restoring the ability of ADP to induce platelet aggregation in the presence of P2Y12 receptor antagonists.25
The activation of PI3-K has been shown to be implicated in platelet aggregation.26 There are 3 families of PI3-K (classes I, II, and III). The class I PI3-K is responsible for agonist-induced PtdIns(3,4)P2 and PtdIns(3,4,5)P3 production and plays a role in the activation of integrin αIIbβ3. The class I PI3-K is subdivided into class IA (α, β, and δ) and class IB (γ) based on their distinct p110 catalytic subunits and modes of regulation.27 The class IA isoforms have p55–85 regulatory subunits and are classically regulated by tyrosine kinases, whereas the class IB isoform has a p101 regulatory subunit and is activated by G protein–coupled receptors.27 Recent studies have reported the selective inhibitors of these PI3-K isoforms.28,,,,–33 Platelets express all class I PI3-K isoforms with lower levels of p110δ.34 It is known that PI3-K γ activation is thought to be mediated by the βγ complexes dissociated from Gi proteins upon receptor activation,35 and exerts a significant role in ADP-induced platelet aggregation.21 It is also shown that PI3-K β has an important role in ADP-induced platelet aggregation.31
Stimulation of platelets with various agonists results in Ser473 and Thr308 phosphorylation of Akt, which results in Akt activation.36 The PI3-K products PtdIns(3,4)P2 and PtdIns(3,4,5)P3 trigger the simultaneous phosphorylation of Akt, and Akt functions as one of the important downstream effectors of PI3-K.37,38 We and others have shown that the Gi-coupled P2Y12 ADP receptor is responsible for a significant proportion of activation of Akt, and ADP-induced Akt phosphorylation requires Gi signaling.22,23
In this study, we have demonstrated a role for IGF-1 and its signaling mechanisms in platelet activation. Because IGF-1 is important for the proliferation and survival of numerous cell types, and PI3-K/Akt pathways are implicated in the survival of various cancer cells, we focused on the role of PI3-K/Akt in IGF-1–mediated signaling in platelets. We found that IGF-1 can potentiate platelet aggregation induced by a number of agonists, including ADP. The potentiating effect of IGF-1 on platelet aggregation occurs in the absence of protein kinase C (PKC) activation or intracellular calcium mobilization and abolished by the presence of the PI3-K inhibitor wortmannin or the PI3-Kα inhibitor PIK-75. IGF-1 induces Akt activation through PI3-Kα by supplementing Gi-dependent pathway in platelets. Therefore, we conclude that IGF-1 potentiates platelet aggregation by synergizing with Gi but not Gq signaling through the PI3-Kα pathway.
Materials and methods
Approval for this study was obtained from the Institutional Review Board of Temple University (Philadelphia, PA). Informed consent was obtained in accordance with the Declaration of Helsinki.
Materials
IGF-1, epinephrine, 2-MeSADP, MRS-2179, apyrase (type V), CDP-Star chemiluminescent substrates, and bovine serum albumin (BSA; fraction V) were purchased from Sigma (St Louis, MO). SFLLRN and AYPGKF were custom synthesized by Invitrogen (Carlsbad, CA). Anti–phospho-Akt (Ser473) antibody was purchased from Cell Signaling Technology (Beverly, MA). Anti-PKCδ antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Alkaline phosphatase (AP)–labeled secondary antibody was from Kirkegaard & Perry Laboratories (Gaithersburg, MD). Wortmannin and LY294002 were from Biomol Research Laboratories (Plymouth Meeting, PA). TGX-221 was purchased from Cayman Chemical (Ann Arbor, MI). PIK-75, AS-252424, and IC87114 were from the lab of Shaun Jackson (Monash University, Victoria, Australia). 5,5′-dimethyl-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (dimethyl BAPTA) was obtained from Molecular Probes (Eugene, OR). AR-C69931MX was gift from AstraZeneca (Loughborough, United Kingdom). Bisindolylmaleimide I (GF 109203X) and AG 1024 were from Calbiochem (San Diego, CA). YM-254890 was a generous gift from Yamanouchi Pharmaceutical (Ibaraki, Japan). All other reagents were reagent grade, and deionized water was used throughout.
Preparation of human platelets
Whole blood was obtained from a pool of healthy volunteers in a one-sixth volume of acid-citrate-dextrose (2.5 g sodium citrate, 1.5 g citric acid, and 2.0 g glucose in 100 mL H2O). Platelet-rich plasma (PRP) was prepared by centrifugation of citrated blood at 230g for 20 minutes at room temperature (RT). Acetylsalicylic acid was added to PRP to a final concentration of 1 mM, and the preparation was incubated for 45 minutes at 37°C followed by centrifugation at 980g for 10 minutes at RT. The platelet pellet was resuspended in Tyrode buffer (138 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, 5 mM glucose, 10 mM HEPES; N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid [pH 7.4], and 0.2% BSA) containing 0.05 U/mL apyrase. This low concentration of apyrase is not enough to block responses to ADP, but will prolong the responsiveness of platelets to ADP by preventing desensitization of the P2Y receptors. These conditions have been standardized in the laboratory. The platelet count was adjusted to 2 × 108 cells/mL.
Platelet aggregation
Platelet aggregation was measured using a lumi-aggregometer (Chrono-Log, Havertown, PA) at 37°C under stirring conditions (900 rpm). A 0.5-mL sample of aspirin-treated washed platelets was stimulated with different agonists, and change in light transmission was measured. Platelets were preincubated with each inhibitor (where noted) as follows: 100 μM MRS-2179, 100 nM AR-C69931MX, 100 μM AG 1024, 10 μM dimethyl-BAPTA, 10 μM GF109203X, 100 nM wortmannin, 25 μM LY294002, 500 nM PIK-75, 500 nM TGX-221, 2 μM AS-252424, 1 μM IC87114, and 50 nM YM-254890 before agonist stimulation in some experiments. The chart recorder (Kipp and Zonen, Bohemia, NY) was set for 0.2 mm/second.
Measurement of Akt phosphorylation
Platelets were stimulated with agonists under nonstirring condition for the appropriate time, and the reaction was stopped by the addition of 3 × sodium dodecyl sulfate (SDS) sample buffer. In some experiments, wortmannin (100 nM), LY294002 (25 μM), PIK-75 (500 nM), TGX-221 (500nM), AS-252424 (2 μM), IC87114 (1 μM), and YM-254890 (50 nM) were added and incubated for 5 minutes at 37°C without stirring before agonist stimulation. Samples were separated on 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride membrane. Nonspecific binding sites were blocked by incubation in Tris-buffered saline/Tween (TBST; 20 mM Tris, 140 mM NaCL, 0.1% [vol/vol] Tween 20) containing 0.5% (wt/vol) milk protein and 3% (wt/vol) BSA for 30 minutes at RT, and membranes were incubated overnight at 4°C with primary antibody (1:1000 in TBST/2% BSA) with gentle agitation. After 3 washes for 5 minutes each with TBST, the membranes were probed with the AP-labeled goat anti-rabbit IgG (1:5000 in TBST/2% BSA) for 1 hour at RT. After additional washing steps, membranes were then incubated with CDP-Star chemiluminescent substrates for 10 minutes at RT, and chemiluminescence was measured using a Fujifilm LAS-3000 Luminescent Image Analyzer (Fuji, Tokyo, Japan).
Results
IGF-1 potentiates 2-MeSADP–induced platelet aggregation
As previously reported,9 IGF-1 alone was unable to induce platelet shape change and aggregation. In contrast, IGF-1 potentiated platelet aggregation induced by a low dose of 2-MeSADP, whereas IGF-1 had no effect on platelet aggregation induced by a higher dose of 2-MeSADP. Figure 1A shows that partial aggregation induced by 30 nM 2-MeSADP was potentiated by increasing concentrations of IGF-1, where the potentiaing effect of IGF-1 was already detectable at 100 nM IGF-1. However, 100 nM 2-MeSADP–induced full aggregation was not affected, even in the presence of a higher concentration of IGF-1 (1 μM). The tyrphostins are a family of synthetic protein tyrosine kinase inhibitors, and AG 1024 has been shown to be highly selective against IGF-1, among other tyrphostins.39 Platelet aggregation induced by 30 nM 2-MeSADP was inhibited by the IGF-1 receptor selective tyrosine kinase inhibitor AG 1024 (Figure 1MB), and the potentiating effect of IGF-1 on 2-MeSADP–induced platelet aggregation was also suppressed by AG 1024. In addition, 100 nM 2-MeSADP–induced full aggregation that was not enhanced by exogenous IGF-1 was not affected by AG 1024 (data not shown). These results demonstrate that IGF-1 potentiates 2-MeSADP–induced platelet aggregation upon its release from α granules.
The effect of IGF-1 on 2-MeSADP–induced platelet aggregation. (A) Aspirin-treated, washed human platelets were stimulated with different concentrations of 2-MeSADP, IGF-1, or the combination of both agonists as noted, and platelet aggregation was measured as described in “Platelet aggregation.” All aggregations tracings were performed in the presence of 1 mg/mL fibrinogen. (B) Platelets were preincubated at 37°C for 10 minutes with 100 μM AG 1024 prior to the stimulation of the platelets. Platelets were stimulated with 30 nM 2-MeSADP or a combination of 30 nM 2-MeSADP and 100 nM IGF-1, as noted. All aggregation tracings are representative of at least 3 experiments from different donors.
The effect of IGF-1 on 2-MeSADP–induced platelet aggregation. (A) Aspirin-treated, washed human platelets were stimulated with different concentrations of 2-MeSADP, IGF-1, or the combination of both agonists as noted, and platelet aggregation was measured as described in “Platelet aggregation.” All aggregations tracings were performed in the presence of 1 mg/mL fibrinogen. (B) Platelets were preincubated at 37°C for 10 minutes with 100 μM AG 1024 prior to the stimulation of the platelets. Platelets were stimulated with 30 nM 2-MeSADP or a combination of 30 nM 2-MeSADP and 100 nM IGF-1, as noted. All aggregation tracings are representative of at least 3 experiments from different donors.
IGF-1 induces platelet aggregation with combined stimulation of Gq, Gi, or Gz pathways
2-MeSADP requires signaling through both Gq-coupled P2Y1 and Gi-coupled P2Y12 receptors to cause platelet aggregation.17 As shown in Figure 2, selective activation of Gq pathways by 2-MeSADP in the presence of the Gi-coupled P2Y12 receptor antagonist AR-C69931MX resulted in platelet shape change. IGF-1 in the presence of Gq-selective stimulation caused platelet aggregation, suggesting the contribution of IGF-1 in platelet aggregation by substituting for Gi-signaling pathways.
Platelet aggregation induced by IGF-1 with combined stimulation of Gq or Gi/Gz pathways. Washed platelets were stimulated with 30 nM 2-MeSADP, 10 μM epinephrine, or a combination of either agonist with 100 nM IGF-1, as noted. Where noted, 100 μM MRS2179 or 100 nM AR-C69931MX were added 1 minute prior to platelet stimulation. All aggregations were performed in the presence of 1 mg/mL fibrinogen. The data shown are representative of at least 3 experiments.
Platelet aggregation induced by IGF-1 with combined stimulation of Gq or Gi/Gz pathways. Washed platelets were stimulated with 30 nM 2-MeSADP, 10 μM epinephrine, or a combination of either agonist with 100 nM IGF-1, as noted. Where noted, 100 μM MRS2179 or 100 nM AR-C69931MX were added 1 minute prior to platelet stimulation. All aggregations were performed in the presence of 1 mg/mL fibrinogen. The data shown are representative of at least 3 experiments.
2-MeSADP (30 nM) was unable to cause platelet aggregation in the presence of Gq-coupled P2Y1 receptor antagonist MRS-2179. However, the combination of Gi stimulation with IGF-1 caused partial platelet aggregation without shape change (Figure 2). It has been shown that a higher dose of ADP causes partial platelet aggregation without shape change in Gq- or P2Y1-deficient mouse platelets, indicating that P2Y12-mediated activation of Gi induces platelet aggregation.40,–42 Thus, these results suggest that IGF-1 causes partial platelet aggregation by enhancing Gi-signaling pathways.
Because it is also possible that potentiation of platelet aggregation induced by IGF-1 can be mediated by complementing Gz-signaling pathways, we tested the effect of IGF-1 with the combination of Gz signaling. We have previously shown that costimulation of Gi and Gz signaling is sufficient to cause aggregation in both human and mouse platelets.43 Selective stimulation of Gz signaling through α2A adrenergic receptor with 10 μM epinephrine did not cause platelet aggregation, but the combination of IGF-1 (100 nM) with epinephrine caused partial platelet aggregation without shape change (Figure 2) in a dose-dependent manner from 100 nM to 1 μM (data not shown). These results are similar to the costimulation of Gi signaling with ADP and Gz signaling with epinephrine,43 confirming that IGF-1 substitutes for Gi-dependent pathways to trigger platelet aggregation in combination with Gz signaling.
IGF-1 potentiates platelet aggregation through PI3-K
In order to identify the intracellular signaling mediators that contribute to the potentiating effect of IGF-1 on platelet aggregation, we tested effects of inhibiting calcium, PKC, and PI3-K on the aggregation induced by IGF-1. As shown in Figure 3A, IGF-1 was able to potentiate 2-MeSADP–induced platelet aggregation in the presence of the pan-PKC inhibitor GF109203X (10 μM) or the intracellular calcium chelator dimethyl BAPTA (10 μM). Despite its potentiating effect on 2-MeSADP–induced platelet aggregation, IGF-1 failed to potentiate platelet aggregation induced by 2-MeSADP in the presence of the PI3-K inhibitor wortmannin (100 nM), suggesting that IGF-1 requires PI3-K activation to induce potentiation of platelet aggregation.
The effect of kinase inhibitors on potentiation of 2-MeSADP–induced platelet aggregation by IGF-1. (A) Washed platelets preincubated with 100 nM wortmannin, 10 μM dimethyl-BAPTA, or 10 μM GF109203X were stimulated at 37°C with either 30 nM 2-MeSADP or a combination of 30 nM 2-MeSADP and 100 nM IGF-1, as noted. (B) Washed platelets preincubated with 100 nM wortmannin, 500 nM PIK-75, 500 nM TGX-221, 2 μM AS-252424, or 1 μM IC87114 were stimulated at 37°C with either 30 nM 2-MeSADP or a combination of 30 nM 2-MeSADP and 100 nM IGF-1, as noted. All aggregations were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 different donors. (C) The percentage of potentiation of 2-MeSADP–induced platelet aggregation by IGF-1 in the presence of specific PI3-K inhibitors shown in panel B is quantified in the bar graphs, and the results are means plus or minus SE (n = 3).
The effect of kinase inhibitors on potentiation of 2-MeSADP–induced platelet aggregation by IGF-1. (A) Washed platelets preincubated with 100 nM wortmannin, 10 μM dimethyl-BAPTA, or 10 μM GF109203X were stimulated at 37°C with either 30 nM 2-MeSADP or a combination of 30 nM 2-MeSADP and 100 nM IGF-1, as noted. (B) Washed platelets preincubated with 100 nM wortmannin, 500 nM PIK-75, 500 nM TGX-221, 2 μM AS-252424, or 1 μM IC87114 were stimulated at 37°C with either 30 nM 2-MeSADP or a combination of 30 nM 2-MeSADP and 100 nM IGF-1, as noted. All aggregations were performed in the presence of 1 mg/mL fibrinogen. Tracings are representative of results obtained from 3 different donors. (C) The percentage of potentiation of 2-MeSADP–induced platelet aggregation by IGF-1 in the presence of specific PI3-K inhibitors shown in panel B is quantified in the bar graphs, and the results are means plus or minus SE (n = 3).
To better understand the role of individual PI3-K isoforms, we have evaluated the effect of specific PI3-K isoforms on IGF-1–mediated potentiation of 2-MeSADP–induced platelet aggregation by using PI3-Kα inhibitor PIK-75, PI3-Kβ inhibitor TGX-221, PI3-Kγ inhibitor AS-252424, and PI3-Kδ inhi-bitor IC87114.30,,–33 IGF-1 failed to potentiate 2-MeSADP–induced platelet aggregation in the presence of PIK-75, whereas IGF-1 was able to potentiate 2-MeSADP–induced platelet aggregation in the presence of TGX-221, AS-252424, or IC87114(Figure 3B,C), indicating the important role of the PI3-Kα isoform in this potentiating event by IGF-1. When platelets were treated with another PI3-Kα inhibitor, compound 15e,28 IGF-1 was also unable to potentiate 2-MeSADP–induced platelet aggregation (data not shown), confirming a critical role for PI3-Kα.
Effect of IGF-1 on SFLLRN-induced platelet aggregation
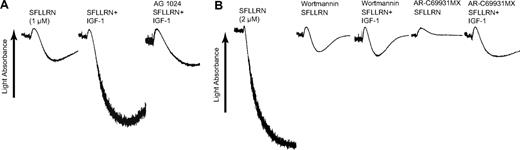
To verify the effect of IGF-1 on platelet function, we have evaluated the effect of IGF-1 on other agonist-induced platelet aggregation. Platelet aggregation induced by low concentrations of the PAR1-activating peptide SFLLRN was potentiated by IGF-1, which was abolished by the presence of AG 1024 (Figure 4A). IGF-1 had no effect on platelet aggregation induced by higher concentrations of SFLLRN (data not shown). Inhibition of PI3-K by wortmannin completely abolished the potentiating effect of IGF-1 on SFLLRN-mediated platelet aggregation (Figure 4B), confirming an important role for PI3-K in IGF-1 signaling. Gi stimulation by secreted ADP has been shown to contribute to low concentrations of PAR agonist-mediated platelet aggregation. IGF-1 was able to potentiate SFLLRN-induced platelet aggregation in the presence of the P2Y12 receptor antagonist AR-C69931MX, indicating that the potentiating effect of IGF-1 on SFLLRN-induced platelet aggregation is independent of secreted ADP. Similar results were observed with the PAR4-activating peptide AYPGKF (data not shown).
The effect of IGF-1 on SFLLRN-induced platelet aggregation. (A) Washed platelets were stimulated with either 1 μM SFLLRN or a combination of 1 μM SFLLRN and 100 nM IGF-1, as noted. Where noted, platelets were preincubated at 37°C for 10 minutes with 100 μM AG 1024. (B) Platelets were stimulated with either 2 μM SFLLRN or a combination of 2 μM SFLLRN and 100 nM IGF-1, as noted. Where noted, AR-C69931MX (100 nM) or wortmannin (100 nM) were added 1 minute prior to platelet stimulation. Results are representative of 3 independent experiments.
The effect of IGF-1 on SFLLRN-induced platelet aggregation. (A) Washed platelets were stimulated with either 1 μM SFLLRN or a combination of 1 μM SFLLRN and 100 nM IGF-1, as noted. Where noted, platelets were preincubated at 37°C for 10 minutes with 100 μM AG 1024. (B) Platelets were stimulated with either 2 μM SFLLRN or a combination of 2 μM SFLLRN and 100 nM IGF-1, as noted. Where noted, AR-C69931MX (100 nM) or wortmannin (100 nM) were added 1 minute prior to platelet stimulation. Results are representative of 3 independent experiments.
Akt phosphorylation in response to IGF-1 in platelets
It is known that Akt functions as 1 of the major downstream effectors of PI3-K,37,38 and PI3-K/Akt plays an important role in the Gi-dependent potentiation of platelet aggregation.22,26 It is also known that ADP-induced Akt phosphorylation requires Gi signaling.22,23 To determine the effect of IGF-1 on Akt phosphorylation, we exposed platelets to different concentrations of IGF-1 ranging from 100 nM to 1 μM, and Akt phosphorylation (Ser473) was measured at 3 minutes after the addition of agonist. IGF-1 induced a concentration-dependent increase in Akt phosphorylation (Figure 5A). A weak Ser473 phosphorylation was detectable at a concentration of 100 nM IGF-1, and higher concentrations revealed increased Akt phosphorylation. In order to determine the potentiating effect of IGF-1 on Akt phosphorylation, we exposed platelets to different concentrations of IGF-1 in the presence of 2-MeSADP. Figure 5B shows that IGF-1 potentiated, in a concentration-dependent manner, Akt phosphorylation mediated by 2-MeSADP. Simultaneous addition of IGF-1 even at the concentration of 100 nM to 2-MeSADP–stimulated platelets resulted in a significant increase in Akt phosphorylation compared with that induced by 2-MeSADP alone, suggesting that IGF-1 is capable of potentiating Akt phosphorylation mediated by Gi pathways.
Akt is phosphorylated in response to IGF-1 in platelets. (A) Washed platelets were stimulated with different concentrations of IGF-1 for 3 minutes at 37°C without stirring. (B) Platelets were stimulated simultaneously with 2-MeSADP (100 nM) and different concentrations of IGF-1 for 3 minutes at 37°C. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with anti-phospho-Akt (Ser473) or anti-PKCδ (lane loading control) antibody. The data shown are representative of 3 experiments.
Akt is phosphorylated in response to IGF-1 in platelets. (A) Washed platelets were stimulated with different concentrations of IGF-1 for 3 minutes at 37°C without stirring. (B) Platelets were stimulated simultaneously with 2-MeSADP (100 nM) and different concentrations of IGF-1 for 3 minutes at 37°C. The reaction was stopped by the addition of 3 × SDS sample buffer. Samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and probed with anti-phospho-Akt (Ser473) or anti-PKCδ (lane loading control) antibody. The data shown are representative of 3 experiments.
Differential effect of PI3-K inhibitors on Akt phosphorylation mediated by IGF-1
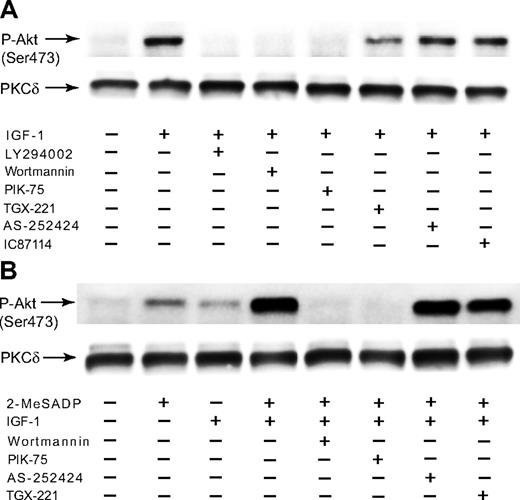
As PI3-K is the main upstream regulator of Akt, we studied the role of PI3-K in IGF-1–induced Akt phosphorylation. We evaluated the contribution of PI3-K isoforms to Akt phosphorylation by using different PI3-K selective inhibitors.30,,–33 It is known that ADP-induced Akt phosphorylation depends on Gi stimulation through PI3-K.22,23 Immunoblot analysis revealed that Akt phosphorylation induced by IGF-1 was completely abolished by the PI3-K inhibitors LY204002 and wortmannin (Figure 6A), confirming the essential role of PI3-K in this event. IGF-1-induced Akt phosphorylation was completely inhibited by the PI3-K p110α inhibitor PIK-75, whereas the PI3-K p110β inhibitor TGX-221, the PI3-K p110γ inhibitor AS-252424, and the PI3-K p110δ inhibitor IC87114 showed little or no effect on Akt phosphorylation induced by IGF-1. It appears that IGF-1 requires PI3-K p110α for Akt phosphorylation. In addition, IGF-1 failed to potentiate Akt phosphorylation mediated by 2-MeSADP in the presence of wortmannin and PIK-75 (Figure 6B), confirming the essential role of PI3-K, especially PI3-K p110α, in the potentiation of 2-MeSADP–mediated Akt phosphorylation by IGF-1.
The effect of PI3-K inhibitors on Akt phosphorylation induced by IGF-1 in platelets. (A) Washed platelets preincubated with 25 μM LY294002, 100 nM wortmannin, 500 nM PIK-75, 500 nM TGX-221, 2 μM AS-252424, or 1 μM IC87114 were stimulated at 37°C for 3 minutes with either 1 μM IGF-1. (B) Platelets preincubated with 100 nM wortmannin, 500 nM PIK-75, 500 nM TGX-221, or 2 μM AS-252424 were stimulated at 37°C for 3 minutes with either 100 nM 2-MeSADP, 100 nM IGF-1, or a combination of 100 nM 2-MeSADP and 100 nM IGF-1, as noted. The reaction was stopped by the addition of 3 × SDS sample buffer. Equal amounts of proteins were analyzed by Western blot analysis with anti-Ser(P)473 or anti-PKCδ (lane loading control) antibody. The Western analysis shown is a representative of 3 independent experiments.
The effect of PI3-K inhibitors on Akt phosphorylation induced by IGF-1 in platelets. (A) Washed platelets preincubated with 25 μM LY294002, 100 nM wortmannin, 500 nM PIK-75, 500 nM TGX-221, 2 μM AS-252424, or 1 μM IC87114 were stimulated at 37°C for 3 minutes with either 1 μM IGF-1. (B) Platelets preincubated with 100 nM wortmannin, 500 nM PIK-75, 500 nM TGX-221, or 2 μM AS-252424 were stimulated at 37°C for 3 minutes with either 100 nM 2-MeSADP, 100 nM IGF-1, or a combination of 100 nM 2-MeSADP and 100 nM IGF-1, as noted. The reaction was stopped by the addition of 3 × SDS sample buffer. Equal amounts of proteins were analyzed by Western blot analysis with anti-Ser(P)473 or anti-PKCδ (lane loading control) antibody. The Western analysis shown is a representative of 3 independent experiments.
IGF-1 potentiates Akt phosphorylation when combined with G12/13 signaling
We have shown that G12/13 signaling has a potentiating effect on Akt phosphorylation when combined with selective Gi stimulation through PI3-K in platelets.44 To confirm the contribution of IGF-1 to Akt phosphorylation, we selectively activated G12/13 signaling with SFLLRN or AYPGKF in the presence of the Gq-selective inhibitor YM-25489045 and measured the effect of combined stimulation of G12/13 and IGF-1 on Akt phosphorylation. As shown in Figure 7, selective stimulation of G12/13 signaling with SFLLRN and AYPGKF in the presence of YM-254890 failed to induce Akt phosphorylation. However, IGF-1 in the presence of YM-254890 restored Akt phosphorylation to the extent achieved by these agonists, confirming that G12/13 signaling potentiates Akt phosphorylation induced by IGF-1 through supplemental Gi signaling.
IGF-1 restores SFLLRN- and AYPGKF-induced Akt phosphorylation upon blockade of Gq pathways in platelets. Aspirin-treated, washed human platelets preincubated with 50 nM YM-254890 were stimulated at 37°C for 3 minutes with either SFLLRN (3 μM) or AYPGKF (200 μM) in the absence and presence of 100 nM IGF-1. The reaction was stopped by the addition of 3 × SDS sample buffer. Platelet proteins were separated by SDS-PAGE, Western-blotted, and probed for anti-phospho-Akt (Ser473) or anti-PKCδ (lane loading control) antibody. The blot shown is a representative of 3 independent experiments.
IGF-1 restores SFLLRN- and AYPGKF-induced Akt phosphorylation upon blockade of Gq pathways in platelets. Aspirin-treated, washed human platelets preincubated with 50 nM YM-254890 were stimulated at 37°C for 3 minutes with either SFLLRN (3 μM) or AYPGKF (200 μM) in the absence and presence of 100 nM IGF-1. The reaction was stopped by the addition of 3 × SDS sample buffer. Platelet proteins were separated by SDS-PAGE, Western-blotted, and probed for anti-phospho-Akt (Ser473) or anti-PKCδ (lane loading control) antibody. The blot shown is a representative of 3 independent experiments.
Discussion
IGF-1 in platelets is important because IGF-1 is released from the platelet immediately after tissue injury directly into the tissue, where additive and synergistic actions with other growth factors in α granules, including platelet-derived growth factor, epidermal growth factor, and transforming growth factor-β, can promote tissue repair.46 The IGF-1 in platelets is important not only as early determinants of wound healing, but could be involved in other platelet functions, such as the maintenance of vascular integrity and atherogenesis.47 Therefore, the primary aim of present study was to determine the functional role of IGF-1 and its intracellular signaling events in platelet activation.
Even though IGF-1 alone fails to induce platelet aggregation, platelet aggregation induced by submaximal concentrations of 2-MeSADP, SFLLRN, and AYPGKF is potentiated by the addition of IGF-1 (100 nM), suggesting that IGF-1 plays an important role in potentiating platelet aggregation. This potentiating effect is observed with a much lower concentration of exogenously added IGF-1, as low as 10 nM (76 ng/mL) IGF-1 (data not shown). The normal physiologic range of IGF-1 in plasma is 150 to 400 ng/mL, suggesting the physiologic relevance of the potentiating effect of IGF-1. ADP has been shown to mediate α granule release in washed human platelets.7 2-MeSADP–induced platelet aggregation and the potentiaing effect of IGF-1 on 2-MeSADP–induced platelet aggregation was inhibited in the presence of AG 1024, suggesting that IGF-1 may have a direct role in platelet function, and IGF-1 secreted from the α granule can potentiate platelet aggregation under physiologic conditions.
ADP-induced platelet aggregation requires coactivation of both the Gq-coupled P2Y1 receptor and the Gi-coupled P2Y12 receptor.17 The P2Y1 receptor is essential for intracellular calcium mobilization and platelet shape change, whereas the P2Y12 receptor leads to inhibition of AC.18,48 Even though selective P2Y1 receptor or P2Y12 receptor activation alone has been shown to be unable to cause platelet aggregation, we found that IGF-1 can trigger platelet aggregation in combination with selective P2Y1 receptor activation, suggesting that IGF-1 supplements Gi-signaling pathways. Our results also show that the combination of IGF-1 and P2Y12 receptor activation triggered platelet aggregation without shape change. It is known that high concentrations of ADP can induce partial platelet aggregation without calcium mobilization or platelet shape change in P2Y1 receptor– or Gq-deficient mouse platelets,41,42 indicating that P2Y12 receptor/Gi signaling can induce this partial platelet aggregation that is dependent on PI3-K.40 In addition, IGF-1 was able to potentiate platelet aggregation in the presence of calcium chelator or PKC inhibitor, indicating that the potentiating effect of IGF-1 on platelet aggregation is independent of intracellular calcium and PKC activation. These results suggest that IGF-1 may exert its potentiating effect on platelet aggregation by synergizing Gi, but not Gq, signaling. While G12/13 signaling does not have a direct effect on Akt phosphorylation, it plays an important role in potentiating Akt phosphorylation mediated by Gi pathways in platelets.44 Selective stimulation of G12/13 signaling significantly increases the Akt phosphorylation in combination with IGF-1 stimulation, further indicating that IGF-1 can supplement Gi-signaling pathways.
Since Gz signaling triggered by epinephrine through the α2A adrenergic receptor can supplement Gi signaling,25 it was necessary to differentiate the effect of IGF-1 on Gz-signaling pathways. Gz signaling alone is not able to cause platelet aggregation.49 It has been shown that combined stimulation of Gi signaling through the P2Y12 receptor and Gz signaling through the α2A adrenergic receptor causes platelet aggregation, where Gi or Gz signaling alone did not cause platelet aggregation.40,43 Epinephrine-mediated signaling through Gz pathways or IGF-1 stimulation per se did not induce platelet aggregation, but the combination of epinephrine and IGF-1 was able to induce platelet aggregation without shape change. These results confirm that IGF-1 triggers platelet aggregation in combination with Gz signaling by selectively supplementing Gi signaling. However, the effect of IGF-1 may not be due to the inhibition of AC through Gi signaling because the platelet aggregation induced by combination of Gi and Gz signaling is independent of inhibition of AC.40
The activation of PI3-K downstream of Gi pathways is implicated in stabilization of platelet aggregation.26 It has been shown that PI3-K activity is required for P2Y12 receptor–mediated platelet aggregation.40 Our results using the PI3-K inhibitor wortmannin show that PI3-K activity is necessary for the potentiating effect of IGF-1 on platelet aggregation. However, it was not clear which subtype of PI3-K (α, β, δ, or γ) is involved in this potentiating effect because of the lack of selectivity of wortmannin on PI3-K subtypes. It is reported that PI3-Kγ exerts a significant role in ADP-induced platelet aggregation.21 It is also shown that PI3-Kβ has an important role in sustaining platelet aggregation in response to weak agonist stimulation.31 To identify the subtype of PI3-K involved in platelet aggregation induced by IGF-1, we have used several PI3-K inhibitors.29,,,–33 Our results show that IGF-1 is unable to potentiate platelet aggregation in the presence of the PI3-K p110α inhibitors PIK-75 and compound 15e, whereas IGF-1 can exert its potentiating effect in the presence of the PI3-K p110β inhibitor TGX-221, the PI3-K p110γ inhibitor AS-252424, or the PI3-K p110δ inhibitor IC87114, indicating the essential role of PI3-K p110α in this potentiating effect by IGF-1. Thus, IGF-1 and 2-MeSADP may use different signaling pathways to activate different isoforms of PI3-K and potentiate each other to induce platelet aggregation.
As PI3-K is known to be an upstream regulator of Akt, one would expect Akt phosphorylation to occur downstream of IGF-1. It has been shown that activation of the P2Y12 receptor leads to Akt activation through IGF-1 receptor cross-talk in C6 glioma cells.10 In fact, stimulation of platelets with IGF-1 resulted in Akt phosphorylation. We and others have reported that ADP depends on the Gi-coupled P2Y12 receptor for Akt activation in platelets, and PI3-K activity is necessary for Akt activation in platelets.22,23 Our results using LY294002 and wortmannin show that PI3-K is required for IGF-1–mediated Akt phosphorylation. However, it is not clear which PI3-K isoforms are involved in Akt activation in platelets. Previous study has shown that Akt phosphorylation induced by ADP is partially inhibited in PI3-Kγ knockout mouse platelets.21 It is also shown that PI3-K p110β is the major regulator of the P2Y12/Gi-signaling process linked to sustained platelet aggregation and Gi activation of Rap1b.31 We have found that Akt phosphorylation induced by IGF-1 was completely inhibited in the presence of PIK-75, whereas TGX-221, AS-252424, or IC87114 had little or no effect on Akt phosphorylation. We have also found that PIK-75 prevented Akt phosphorylation induced by costimulation of IGF-1 and 2-MeSADP. These results suggest that IGF-1 and 2-MeSADP may use different signaling events that synergize to Akt activation, and IGF-1 depends on PI3-K p110α to induce Akt phosphorylation. Thus, IGF-1 may require PI3-K p110α to cause PtdIns(3,4,5)P3 production, and PtdIns(3,4,5)P3 production downstream of IGF-1 is abolished in the absence of PI3-K p110α, which results in the complete inhibition of Akt phosphorylation.
In conclusion, we demonstrate that IGF-1 potentiates platelet aggregation induced by low concentrations of agonists, including 2-MeSADP. These effects occur in the absence of intracellular Ca2+ increase or PKC activation. IGF-1 induces Akt phosphorylation through PI3-K p110α and mediates its potentiating effect by supplementing Gi signaling through PI3-K p110α pathways.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research grants HL60683 and HL80444 from the National Institutes of Health (NIH; to S.P.K.) and by NIH training grant HL00777 in thrombosis (to A. G.).
National Institutes of Health
Authorship
Contribution: S.K. designed and performed experiments, analyzed data, and wrote the paper. A.G. performed experiments. S.P.J. contributed vital new reagents and analyzed data. S.P.K. was responsible for the overall direction, designed experiments, and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satya P. Kunapuli, Department of Physiology, Temple University School of Medicine, 3420 N Broad St, Philadelphia, PA 19140; e-mail:spk@temple.edu.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal