Plasmacytoid dendritic cells (pDCs), also known as type I interferon (IFN)–producingcells, are thought to play central roles in antiviral immunity and the pathogenesis of some autoimmune diseases. pDCs are produced from hematopoietic stem cells in bone marrow. However, the environmental regulation of the development of pDCs is not fully understood. Here, we show that the numbers of pDCs and their earliest progenitors are severely reduced in the absence of CXCR4, the primary physiologic receptor for CXC chemokine ligand 12 (CXCL12), also known as stromal cell–derived factor-1 (SDF-1) in vivo. In vitro, CXCL12 induces a significant increase in pDC numbers generated from primitive hematopoietic cells, and pDCs and their progenitors migrate to CXCL12. In addition, most pDCs are in contact with CXCL12-abundant reticular (CAR) cells in the intersinal space of bone marrow, although many primitive hematopoietic cells adjoin CAR cells surrounding sinusoidal endothelial cells or residing near the bone surface. Thus we identified CXCL12 as a key regulator of pDC development produced by cellular niches, providing new targets for pDC therapeutic control.
Introduction
Plasmacytoid dendritic cells (pDCs), also known as type I interferon (IFN)–producing cells (IPCs), are a specialized cell population that display plasma cell morphology and rapidly produces a huge amount of type I IFN (α, β) following microbial stimulation.1,2 pDCs selectively express intracellular Toll-like receptor 7 (TLR7) and TLR9, and are thought to be the most important cell type in antiviral innate immunity.1,2 In addition, pDCs can link innate with adaptive immunity through type I IFN during an antimicrobial immune response.1,2 It is thought that pDCs are produced constantly from hematopoietic stem cells (HSCs), which give rise to all types of blood cells, in bone marrow and migrate to the spleen, lymph nodes, and mucosal-associated lymphoid tissues during adult life. However, regulation of the development of pDCs by environmental factors is not fully understood.1,–3 Among the known cytokines, only Flt3 ligand (Flt3L; also known as Flk2 ligand) has been shown to be essential for pDC development.4 Flt3L is a ligand for Flt3 (also known as Flk2), which has sequence and structural homology to the class III receptor tyrosine kinases, including the stem cell factor (SCF) receptor (c-kit). It has been shown that Flt3L-deficient mice have reduced numbers of splenic CD11c+B220+ pDCs and CD11c+B220− conventional dendritic cells (cDCs) compared with wild-type mice.4 In addition, the numbers of cells in Fraction A subset, which contain both the earliest B-cell precursors and pDCs in bone marrow5,6 are severely reduced in the mutants, accompanied by modest reduction in mature B cells in the bone marrow and spleen.4,7,8 Flt3L promotes the generation of pDCs and cDCs in vitro and in vivo,9,–11 and overexpression of Flt3 in Flt3− hematopoietic progenitors rescues their pDC and cDC differentiation potential.12 Interleukin-7 (IL-7) and its receptor (IL-7Rα) are known to be essential for B- and T-cell development. The numbers of CD11c+B220+ pDCs are unaffected in bone marrow but are reduced in the spleen by the absence of IL-7Rα.5,13,14 However, IL-7Rα–deficient mice have relatively normal IFN-α production in their splenocytes, suggesting that IL-7 is not essential for the generation of functional pDCs.5,13,14
CXC chemokine ligand 12 (CXCL12; also known as stromal cell–derived factor-1 [SDF-1]/pre–B-cell growth-stimulating factor [PBSF]) is a member of chemokines, which are a large family of structurally related chemoattractive cytokines, and its primary physiologic receptor is CXCR4, a 7-transmembrane–spanning G protein–coupled receptor, which also functions as an entry receptor for strains of HIV-1.15,,–18 CXCL12 was first characterized as a growth-stimulating factor for B-cell precursors,19 and the studies using mutant mice with targeted gene disruption have revealed that CXCL12-CXCR4 signaling is essential for colonization of bone marrow by hematopoietic cells, including HSCs during ontogeny, maintenance of the HSC pool in adult bone marrow, and B-cell development, as well as cardiovascular formation and neurogenesis.15,,–18,20,–22 Of particular note, the study using radiation chimeras reconstituted with fetal liver cells have shown that the numbers of cells in Fraction A were severely reduced in the absence of CXCR4,20 raising the possibility that CXCL12-CXCR4 signaling is involved in pDC development.
In this study, we examined the roles of CXCL12-CXCR4 signaling in pDC development in vivo and in vitro and have found that CXCL12 plays an essential role at a very early stage of pDC development.
Materials and methods
Mice
CXCR4−/− mice,17 CXCL12/GFP knock-in mice,21 and CXCR4flox(f)/null mice23 were maintained on a C57BL/6-Ly5.2+/Ly5.1− background. For the generation of fetal liver–chimeric mice, 4 × 106 fetal liver cells from CXCR4+/− or CXCR4−/− embryos at embryonic day 16.5 (E16.5) were transferred intravenously into lethally irradiated C57BL/6-Ly5.2−/Ly5.1+ recipient mice as described previously.20,24 The chimeric mice were analyzed 16 to 45 weeks after transfer. CXCR4f/null mice were crossed with MxCre transgenic mice to generate Cre-mediated CXCR4 conditionally deficient mice as described.22 The MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice were treated with poly(I)-poly(C) (pIpC; Sigma, St Louis, MO), and analyzed at 1 week or 3 weeks after the final pIpC treatment. For the generation of bone marrow–chimeric mice, 2 × 106 bone marrow cells from MxCre/CXCR4f/wt or MxCre/CXCR4f/null mice carrying Ly5.2+/Ly5.1− alleles were mixed with 1 × 106 bone marrow cells from C57BL/6-Ly5.2−/Ly5.1+ mice as competitor cells and were transplanted into lethally irradiated C57BL/6-Ly5.2−/Ly5.1+ recipient mice. At 13 weeks after transplantation, recipients were treated with pIpC, and analyzed at 10 weeks after the final pIpC treatment.22 All animal experimentation was conducted in accordance with the guidance of the Institute for Frontier Medical Sciences, Kyoto University.
Antibodies
The monoclonal antibodies used in immunofluorescence included HL3 (anti-CD11c), RA3-6B2 (anti-B220), 1D3 (anti-CD19), PK136 (anti-NK1.1), GK1.5 (anti-CD4), 53–6.7 (anti-CD8α), 2B8 (anti–c-kit), M1/70 (anti-CD11b), 2.4G2 (anti-FcγRII/III), AF6–120.1 (anti–I-Ab MHC II; BD Biosciences, San Jose, CA), A2F10 (anti-Flt3), D7 (anti–Sca-1), E13-161.7 (anti–Sca-1), A7R34 (anti–IL-7Rα; eBioscience, San Diego, CA), JF05-1C2.4.1 (anti–PDCA-1; Miltenyi Biotec, Bergisch Gladbach, Germany), and RMMA-1 (anti–IFN-α; PBL Biomedical Laboratories, Piscataway, NJ). Anti–IFN-α was biotinylated using the Biotin Labeling Kit (Dojindo, Kumamoto, Japan) following the manufacturer's instructions. Lineage (Lin) antibodies included anti-B220, anti-CD11b, TER119 (anti-erythrocyte–specific antigen), RB6–8C5 (anti–Gr-1), and 145-2C11 (anti-CD3ϵ). A20 (anti-Ly5.1) and 104 (anti-Ly5.2) were used to distinguish donor cells from recipient cells. Affinity-purified antibodies were detected with goat anti-rat Ig, and biotinylated antibodies were detected with streptavidin-conjugated PE-Cy7 (BD Biosciences), Alexa Fluor 647, or Pacific Blue (Invitrogen, Carlsbad, CA). The monoclonal antibodies used to enrich Lin− cells included anti-erythrocyte–specific antigen, 17A2 (anti-CD3), 1A8 (anti-Ly–6G), DX5 (anti-CD49b; BD Biosciences), and 6D5 (anti-CD19; eBioscience).
Flow cytometry
Bone marrow from tibiae and femurs and spleens were harvested and digested in a solution containing 400 U/mL collagenase D (Roche, Basel, Switzerland) for 30 minutes at 37°C. The resulting single-cell suspensions were then washed and stained with monoclonal antibodies and its secondary reagents. Cells were analyzed or sorted on a FACSAria (BD Biosciences). Donor-derived pDCs were defined as Ly5.2+Ly5.1−CD11cintB220+PDCA-1+CD19−NK1.1− cells. Propidium iodide (Dojindo) was used to distinguish dead cells from viable cells.
For intracellular IFN-α staining, single-cell suspensions from bone marrow or spleen were incubated at 2 × 107 cells/mL in 96-well plate in RPMI 1640 (Sigma) supplemented with 10% fetal calf serum (FCS; SAFC Biosciences, Lenexa, KS), 1% sodium pyruvate, 1% nonessential amino acids (Invitrogen), 50 μM 2-mercaptoethanol (Sigma), and 0.2 μg/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ). Polyuridylic acid (poly U; Sigma) was used at 40 μg/mL, as complexes with jetPEI transfection reagent (Polyplus Transfection, San Marcos, CA).25 CpG-ODN 2216 (ggG GGA CGA TCG TCg ggg gG; Hokkaido System Science, Hokkaido, Japan) was used at 5 μM. Small letters indicate phosphorothioate linkage. After 3 hours of incubation, 1 μL/mL GoldiPlug (BD Biosciences) was added and incubated for additional 6 hours. Subsequently, cells were harvested, washed, and stained with antibodies against surface markers. Fixation and permeabilization were performed with Cytofix/Cytoperm solution (BD Bioscience), followed by intracellular staining with biotinylated anti–IFN-α and its secondary reagent, streptavidin-conjugated Pacific Blue. Ethidium monoazide bromide (Invitrogen) was used for exclusion of dead cells.
ELISA
For detection of IFN-α in culture supernatants, donor-derived Ly5.2+/Ly5.1− sorted cells in bone marrow from fetal liver–chimeric mice were cultured at 1 × 106 cells/mL in 96-well culture plates in RPMI 1640 with 10% FCS, 1% sodium pyruvate, 1% nonessential amino acids, and 50 μM 2-mercaptoethanol. CpG-ODN 2216 was used at 5 μM. After 20 hours of culture, concentrations of IFN-α in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA; PBL Biomedical Laboratories).
In vitro culture of primitive hematopoietic cells and pDC progenitors
Sorted Lin−Sca-1+c-kit+Flt3loIL-7R− cells (440-1000/well) and cells in the Flt3+ subset of common lymphoid progenitor (CLP) and common myeloid progenitor (CMP) fractions (300-880/well) from bone marrow were cultured in Opti-MEM (Invitrogen) with 10% FCS containing 50 μM 2-mercaptoethanol. Flt3L, SCF, IL-7 (R&D Systems, Minneapolis, MN), and CXCL12 were used at 100 ng/mL, 20 ng/mL, 1 ng/mL, and 1 μg/mL, respectively. The numbers of CD11cintB220+PDCA-1+CD11b−CD19− pDCs and CD11chiB220−CD11b+ cDCs were measured by flow cytometry at 4, 8, and 14 days of culture.
Chemotaxis assay
Bone marrow cells were incubated with Lin antibodies described here, followed by negative selection using Dynabeads anti-rat IgG (Invitrogen). These lineage-depleted cells were transferred onto the upper layer of 5-μm-pore polycarbonate membranes (Transwell; Corning, Cambridge, MA), which were then placed into lower chamber containing 100 ng/mL CXCL12, and incubated at 37°C. After 2 hours, cells were harvested from lower chambers, and the numbers of Lin−Sca-1+c-kit+Flt3lo cells, cells in Flt3+ subset of CLP and CMP fractions, and pDCs were counted by flow cytometry.
Immunohistochemical analysis of bone marrow sections
Bone marrow sections from CXCL12/GFP knock-in mice, pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt, and CXCL12/GFP/MxCre/CXCR4f/null mice were analyzed by immunofluorescence as described previously.22 Briefly, 8-μm-thick 4% paraformaldehyde-fixed frozen sections were first blocked with 5% FCS/PBS, and stained with anti–PDCA-1 conjugated with Alexa Fluor 555 by antibody labeling kit (Invitrogen) and biotinylated anti–PECAM-1. Biotinylated antibody was visualized with streptavidin-Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA). Confocal microscopy was performed on a LSM 510 META using a 40×/1.3 NA oil immersion objective lens (Carl Zeiss, Oberkochen, Germany). All acquired images were processed with the LSM Image Browser (Carl Zeiss).
Statistics
Data are expressed as means plus or minus SD. The significance of the differences between groups in the experiments was evaluated by the 2-tailed Student t test.
Results
pDCs are severely reduced in the absence of CXCR4
Since CXCL12−/− or CXCR4−/− mice are embryonic lethal, we first analyzed the chimeric mice reconstituted with CXCR4+/− and CXCR4−/− fetal liver cells to determine the role of CXCL12-CXCR4 signaling in pDC development in adult animals. We transferred fetal liver cells from E16.5 CXCR4+/− and CXCR4−/− mice carrying Ly5.2+/Ly5.1− alleles into lethally irradiated Ly5.2−/Ly5.1+ mice.20,24 We can quantitate the contribution of donor-derived cells in chimeric mice by the cell-surface expression of Ly5.2 and Ly5.1. Mouse pDCs are enriched as CD11cintB220+ cells26 and PDCA-1 antibody selectively recognizes mouse pDCs in bone marrow and lymphoid tissues.27 Thus, we used a CD11cintB220+PDCA-1+ surface phenotype to define pDCs. Flow cytometric analysis revealed that the numbers of donor-derived Ly5.2+CD11cintB220+PDCA-1+ pDCs were severely reduced in bone marrow and spleen in CXCR4−/− chimeras compared with CXCR4+/+ (data not shown) and CXCR4+/− chimeras (Figure 1A,B) at 16 to 45 weeks after transfer, indicating that CXCL12-CXCR4 signaling is essential for the development of pDCs. To confirm this, we analyzed the ability of donor-derived cells in these chimeric mice to produce IFN-α in response to ligands for TLR7 or TLR9. We stimulated donor-derived cells from the bone marrow and spleen of CXCR4−/− and control chimeras with single-stranded RNA (poly U; TLR7 ligand) or CpG-ODN (TLR9 ligand)2 and analyzed their intracellular IFN-α content and IFN-α secretion into supernatants. Flow cytometric analysis revealed that donor-derived cells that secreted IFN-α were detectable in the bone marrow and spleen of CXCR4+/− chimeras, but were almost absent in CXCR4−/− chimeras (Figure 1C). Consistent with this, CpG-ODN treatment induced robust IFN-α secretion into supernatants by donor-derived cells in the bone marrow of control chimeras but failed to induce substantial responses by donor-derived cells in CXCR4−/− chimeras (Figure 1D). These results indicate that CXCL12-CXCR4 signaling is essential for the development of the cells that produce IFN-α in response to ligands for TLR7 and TLR9 (ie, functional pDCs).1
Dramatic reduction of pDCs in the absence of CXCR4. E16.5 fetal liver cells (Ly5.2+/Ly5.1−) from CXCR4+/− and CXCR4−/ − mice were transferred into lethally irradiated Ly5.2−/Ly5.1+ recipients. CXCR4+/− and CXCR4−/ − chimeric mice were analyzed 16 to 45 weeks after transfer. (A-C) Flow cytometric analysis of bone marrow and spleen. Fluorescence staining profile of Ly5.2+CD19−NK1.1− cells (A), and the numbers of donor-derived Ly5.2+CD11cintB220+PDCA-1+ pDCs (n = 4-5) and Ly5.2+CD11chiB220− cDCs (B). (C) Intracellular IFN-α was measured by flow cytometry gating on donor-derived Ly5.2+/Ly5.1−CD19− cells with or without activation with poly U or CpG-ODN. (D) IFN-α production analysis by ELISA on supernatants from donor-derived Ly5.2+/Ly5.1− sorted cells in bone marrow with or without activation with CpG-ODN (n = 3). Error bars represent SD of the mean.
Dramatic reduction of pDCs in the absence of CXCR4. E16.5 fetal liver cells (Ly5.2+/Ly5.1−) from CXCR4+/− and CXCR4−/ − mice were transferred into lethally irradiated Ly5.2−/Ly5.1+ recipients. CXCR4+/− and CXCR4−/ − chimeric mice were analyzed 16 to 45 weeks after transfer. (A-C) Flow cytometric analysis of bone marrow and spleen. Fluorescence staining profile of Ly5.2+CD19−NK1.1− cells (A), and the numbers of donor-derived Ly5.2+CD11cintB220+PDCA-1+ pDCs (n = 4-5) and Ly5.2+CD11chiB220− cDCs (B). (C) Intracellular IFN-α was measured by flow cytometry gating on donor-derived Ly5.2+/Ly5.1−CD19− cells with or without activation with poly U or CpG-ODN. (D) IFN-α production analysis by ELISA on supernatants from donor-derived Ly5.2+/Ly5.1− sorted cells in bone marrow with or without activation with CpG-ODN (n = 3). Error bars represent SD of the mean.
It has been reported previously that pDCs can differentiate into cells with mature cDC morphology in culture.1,28 cDCs are the most powerful antigen-presenting cells, which extend large processes or veils and play a key role in triggering T-cell–dependent immune responses.29 cDCs are identified as CD11chiB220−, well segregated from CD11cintB220+ pDCs,26 and flow cytometric analysis revealed that the numbers of donor-derived Ly5.2+CD11chiB220− cDCs in the spleen of CXCR4−/− chimeras were reduced compared with control chimeras, but to a lesser extent than Ly5.2+CD11cintB220+ pDCs (Figure 1A,B). This suggests that CXCL12-CXCR4 signaling is involved in development of both pDCs and DCs, but plays a specific role in control of pDC development.
The early progenitors of pDCs are severely reduced in the absence of CXCR4
The developmental pathway for early pDC lineage differentiation is not fully understood. It has been shown previously that Flt3+ subsets of Lin−IL-7R+Sca-1loc-kit+ CLP and Lin−Sca-1−c-kit+CD34+ CMP fractions contain early pDC precursors, which can differentiate into pDCs in cultures and in vivo.10,11,30 Flow cytometric analysis of the bone marrow of CXCR4−/− chimeric mice revealed a modest reduction in the numbers of donor-derived Lin−Sca-1+c-kit+Flt3lo cells, which are enriched for primitive hematopoietic cells, including HSCs and multipotent hematopoietic progenitors31 (Figure 2A), but a dramatic reduction of donor-derived cells in Flt3+ subsets of Ly5.2+Lin−IL-7R+Sca-1loc-kit+ CLP and Ly5.2+Lin−Sca-1−c-kit+CD34+ CMP fractions compared with control chimeras at 16 to 45 weeks after transfer (Figure 2B). In addition, CD11c+MHC II− cells, called immediate DC precursors (DCp's), have been suggested to be intermediates between Flt3+ subsets of the CLP or CMP fraction and pDCs or cDCs in murine bone marrow.32 Flow cytometric analysis revealed that donor-derived Ly5.2+CD11c+MHC II− DCp's were almost absent in CXCR4−/− chimeric mice (Figure 2C). These results strongly suggest that CXCL12-CXCR4 signaling plays a critical role in the earliest stages of pDC development.
Dramatic reduction of cells in Flt3+ subsets of CLP and CMP fractions and DCps in the absence of CXCR4. E16.5 fetal liver cells (Ly5.2+/Ly5.1−) from CXCR4+/− and CXCR4−/− mice were transferred into lethally irradiated Ly5.2−/Ly5.1+ recipients. Flow cytometric analysis of the numbers of donor-derived Ly5.2+Lin−Sca-1+c-kit+ (LSK) Flt3lo primitive hematopoietic cells (A); progenitors of pDCs, including Ly5.2+Lin−IL-7R+Sca-1loc-kit+Flt3+ (Flt3+ CLP) and Ly5.2+Lin−Sca-1−c-kit+CD34+Flt3+ (Flt3+ CMP) cells; (B) and Ly5.2+CD11c+MHC II−CD4−CD8−CD19−NK1.1− DCp's (C) in the bone marrow of CXCR4+/− and CXCR4−/− chimeric mice 16 to 45 weeks after transfer (n = 3,5). Error bars represent SD of the mean.
Dramatic reduction of cells in Flt3+ subsets of CLP and CMP fractions and DCps in the absence of CXCR4. E16.5 fetal liver cells (Ly5.2+/Ly5.1−) from CXCR4+/− and CXCR4−/− mice were transferred into lethally irradiated Ly5.2−/Ly5.1+ recipients. Flow cytometric analysis of the numbers of donor-derived Ly5.2+Lin−Sca-1+c-kit+ (LSK) Flt3lo primitive hematopoietic cells (A); progenitors of pDCs, including Ly5.2+Lin−IL-7R+Sca-1loc-kit+Flt3+ (Flt3+ CLP) and Ly5.2+Lin−Sca-1−c-kit+CD34+Flt3+ (Flt3+ CMP) cells; (B) and Ly5.2+CD11c+MHC II−CD4−CD8−CD19−NK1.1− DCp's (C) in the bone marrow of CXCR4+/− and CXCR4−/− chimeric mice 16 to 45 weeks after transfer (n = 3,5). Error bars represent SD of the mean.
Development of pDC progenitors, pDCs, and cDCs in CXCR4 conditionally deficient mice
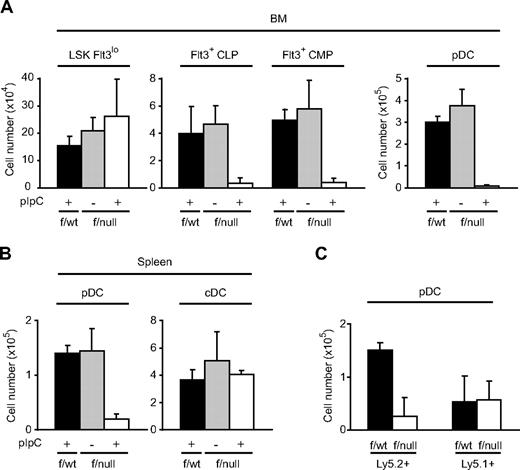
We next analyzed the roles of CXCL12-CXCR4 signaling in pDC development using CXCR4 conditionally deficient mice. We crossed the mice with a loxP-CXCR4 conditional targeting allele (CXCR4f/null mice) to MxCre mice in which Cre was expressed after the induction of IFN by the administration of pIpC (TLR3 ligand) to inactivate the CXCR4 gene in the adult animals.22 The floxed allele was excised almost completely in bone marrow Lin−c-kit+ hematopoietic cells and myeloid lineage cells of some pIpC-treated MxCre/CXCR4f/null mice as analyzed by flow cytometry (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). Flow cytometric analysis revealed that the numbers of Lin−Sca-1+c-kit+Flt3lo primitive hematopoietic cells appeared normal, but the number of Lin−IL-7R+Sca-1loc-kit+Flt3+ (Flt3+ CLP) and Lin−Sca-1−c-kit+CD34+Flt3+ (Flt3+ CMP) pDC progenitors and B220+PDCA-1+ pDCs were severely reduced in the bone marrow of pIpC-treated MxCre/CXCR4f/null mice compared with untreated MxCre/CXCR4f/null mice and control pIpC-treated MxCre/CXCR4f/wt mice 3 weeks after the final pIpC treatment (Figure 3A). In the spleen, the numbers of B220+PDCA-1+ pDCs were severely reduced, but the numbers of CD11chiB220− cDCs appeared normal in the absence of CXCR4 (Figure 3B). Flow cytometric analysis revealed that the floxed allele was excised almost completely in CD11chiB220− cDCs in the spleens of pIpC-treated MxCre/CXCR4f/null mice (Figure S1B).
Development of pDC progenitors, pDCs, and cDCs in CXCR4 conditionally deficient mice. (A,B) Flow cytometric analysis of the numbers of LSK Flt3lo primitive hematopoietic cells, Flt3+ CLP, Flt3+ CMP pDC progenitors, and CD11cintB220+PDCA-1+ pDCs in bone marrow (n = 3) (A); and CD11cintB220+PDCA-1+ pDCs and CD11chiB220− cDCs in the spleen (B) in pIpC-treated MxCre/CXCR4f/wt mice, untreated MxCre/CXCR4f/null mice, and pIpC-treated MxCre/CXCR4f/null mice 3 weeks after the final pIpC treatment. (C) Bone marrow cells (Ly5.2+) from MxCre/CXCR4f/null or MxCre/CXCR4f/wt mice were transferred along with wild-type bone marrow cells (Ly5.1+) into lethally irradiated Ly5.1+ recipients. Chimeric mice were treated with pIpC at 13 weeks after transfer, and their bone marrow was analyzed by flow cytometry at 10 weeks after the treatment. The numbers of Ly5.2+ and Ly5.1+ CD11cintB220+PDCA-1+ pDCs are shown (n = 2). Error bars represent SD of the mean.
Development of pDC progenitors, pDCs, and cDCs in CXCR4 conditionally deficient mice. (A,B) Flow cytometric analysis of the numbers of LSK Flt3lo primitive hematopoietic cells, Flt3+ CLP, Flt3+ CMP pDC progenitors, and CD11cintB220+PDCA-1+ pDCs in bone marrow (n = 3) (A); and CD11cintB220+PDCA-1+ pDCs and CD11chiB220− cDCs in the spleen (B) in pIpC-treated MxCre/CXCR4f/wt mice, untreated MxCre/CXCR4f/null mice, and pIpC-treated MxCre/CXCR4f/null mice 3 weeks after the final pIpC treatment. (C) Bone marrow cells (Ly5.2+) from MxCre/CXCR4f/null or MxCre/CXCR4f/wt mice were transferred along with wild-type bone marrow cells (Ly5.1+) into lethally irradiated Ly5.1+ recipients. Chimeric mice were treated with pIpC at 13 weeks after transfer, and their bone marrow was analyzed by flow cytometry at 10 weeks after the treatment. The numbers of Ly5.2+ and Ly5.1+ CD11cintB220+PDCA-1+ pDCs are shown (n = 2). Error bars represent SD of the mean.
To examine the consequence of CXCR4 deficiency on hematopoietic cells in bone marrow, we generated mice with mixed chimeric bone marrow cells. Bone marrow cells derived from MxCre/CXCR4f/null or MxCre/CXCR4f/wt mice carrying Ly5.2+/Ly5.1− alleles were mixed with wild-type Ly5.2−/Ly5.1+ competitor marrow cells and transferred into lethally irradiated Ly5.2−/Ly5.1+ mice. Analysis of the surface expression of Ly5.2 and Ly5.1 by flow cytometry revealed that donor-derived MxCre/CXCR4f/null and MxCre/CXCR4f/wt cells contributed comparably to peripheral blood cells in these recipient mice 12 weeks after transfer. Mice were then treated with pIpC to induce excision of the floxed allele 13 weeks after transfer. At 10 weeks after the final pIpC treatment, bone marrow was analyzed by flow cytometry. Donor-derived Ly5.2+B220+PDCA-1+ pDCs were severely reduced in mutant chimeras compared with control chimeras, but competitor-derived Ly5.1+B220+PDCA-1+ pDCs in mutant chimeras were not impaired (Figure 3C), suggesting that the deficits in pDC development in bone marrow in the absence of CXCR4 are hematopoietic cell autonomous.
These findings are consistent with the results from experiments using the chimeric mice reconstituted with CXCR4-deficient fetal liver hematopoietic cells, supporting the idea that CXCL12-CXCR4 signaling plays a specific role in the control of pDC development in bone marrow.
In vitro activities of CXCL12 on generation of pDCs from primitive hematopoietic cells
To understand the mechanisms by which CXCL12 affects pDC development, we examined the in vitro activities of CXCL12 in the generation of pDCs from Lin−Sca-1+c-kit+Flt3lo primitive hematopoietic cells and pDC progenitors, including cells in Flt3+ subsets of CLP and CMP fractions isolated from bone marrow. Previous in vitro studies have shown that pDCs are produced from their progenitors in the presence of cytokines, including Flt3L, SCF, and IL-7, which support the survival or proliferation of pDC progenitors.5,9 Cells sorted in Lin−Sca-1+c-kit+Flt3lo cells and Flt3+ subsets of CLP and CMP fractions were cultured in medium containing Flt3L, SCF, and IL-7 in the absence or presence of CXCL12 and the numbers of CD11cintB220+PDCA-1+ pDCs and CD11chiB220− cDCs were measured at 4, 8, and 14 days. Although CD11cintB220+PDCA-1+ pDCs were produced from Lin−Sca-1+c-kit+Flt3lo cells without CXCL12 addition, CXCL12 induced a significant increase in the numbers of pDCs generated from Lin−Sca-1+c-kit+Flt3lo cells after 14 days of culture (Figure 4A). In contrast, CXCL12 had no stimulatory effect on the pDC numbers generated from cells in Flt3+ subsets of CLP and CMP fractions after 4 days of culture (Figure 4B). In addition, CXCL12 had no stimulatory effect on the pDC numbers generated from Lin−Sca-1+c-kit+Flt3lo cells isolated from bone marrow of pIpC-treated MxCre/CXCR4f/null mice (Figure 4A). On the other hand, CD11chiB220− cDCs were generated from Lin−Sca-1+c-kit+Flt3lo cells without CXCL12 addition and CXCL12 induced an increase in cDC numbers, but to a lesser extent than pDC numbers after 14 days of culture (Figure 4C). In the absence of Flt3L, neither CD11cintB220+PDCA-1+ pDCs nor CD11chiB220− cDCs were generated from Lin−Sca-1+c-kit+Flt3lo cells or cells in Flt3+ subsets of CLP and CMP fractions in medium containing SCF, IL-7, and CXCL12 (Figure 4A-C). These results support the idea that CXCL12 acts on Lin−Sca-1+c-kit+Flt3lo primitive hematopoietic cells to support the generation of pDCs.
In vitro activities of CXCL12 in pDCs and their progenitors. (A-C) Effects of CXCL12 on the generation of pDCs (A,B) or cDCs (C) from primitive hematopoietic cells in wild-type mice (A,C) and pIpC-treated MxCre/CXCR4f/null mice 3 weeks after the final pIpC treatment (A), and from more mature pDC progenitors in wild-type mice (B). Cells sorted in LSK Flt3lo cells (A,C) and Flt3+ subsets of CLP and CMP fractions (B) were cultured in medium containing SCF and IL-7 (−Flt3L) or SCF, IL-7, and Flt3L (+Flt3L) in the absence or presence of CXCL12. The numbers of CD11cintB220+PDCA-1+ pDCs (A,B) and CD11chiB220−CD11b+ cDCs (C) measured at 4 days (B) or 14 days (A,C) by flow cytometry are shown (n = 3-6). (D) Chemotactic activity of pDC progenitors toward CXCL12. Transwell migration assay of LSK Flt3lo cells, cells in Flt3+ subsets of CLP and CMP fractions, and CD11cintB220+PDCA-1+ pDCs without CXCL12 or with CXCL12 (n = 3). Error bars represent SD of the mean.
In vitro activities of CXCL12 in pDCs and their progenitors. (A-C) Effects of CXCL12 on the generation of pDCs (A,B) or cDCs (C) from primitive hematopoietic cells in wild-type mice (A,C) and pIpC-treated MxCre/CXCR4f/null mice 3 weeks after the final pIpC treatment (A), and from more mature pDC progenitors in wild-type mice (B). Cells sorted in LSK Flt3lo cells (A,C) and Flt3+ subsets of CLP and CMP fractions (B) were cultured in medium containing SCF and IL-7 (−Flt3L) or SCF, IL-7, and Flt3L (+Flt3L) in the absence or presence of CXCL12. The numbers of CD11cintB220+PDCA-1+ pDCs (A,B) and CD11chiB220−CD11b+ cDCs (C) measured at 4 days (B) or 14 days (A,C) by flow cytometry are shown (n = 3-6). (D) Chemotactic activity of pDC progenitors toward CXCL12. Transwell migration assay of LSK Flt3lo cells, cells in Flt3+ subsets of CLP and CMP fractions, and CD11cintB220+PDCA-1+ pDCs without CXCL12 or with CXCL12 (n = 3). Error bars represent SD of the mean.
It has been reported previously that CXCR4 is expressed in murine DCps33 and human pDCs,34,35 and that human pDCs migrate to CXCL12 in chemotaxis assays.34,35 Quantitative, real-time polymerase chain reaction with reverse transcription (qRT-PCR) analysis revealed that CXCR4 was expressed in Lin−Sca-1+c-kit+Flt3lo primitive hematopoietic cells, cells in Flt3+ subsets of CLP and CMP fractions, and CD11cintB220+PDCA-1+ pDCs in bone marrow of wild-type mice (data not shown). In addition, Lin-Sca-1+c-kit+Flt3lo cells, pDC progenitors, including cells in Flt3+ subsets of CLP and CMP fractions and pDCs migrated to CXCL12 in chemotaxis assays (Figure 4D).
pDCs are associated with CAR cells in the intersinal space of bone marrow
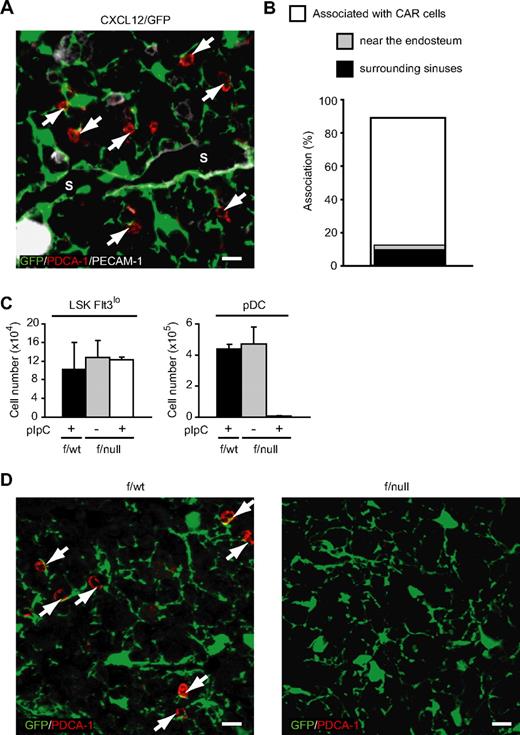
As CXCL12-CXCR4 signaling is essential for pDC development, we were prompted to analyze association of pDCs with a population of reticular cells that expressed CXCL12 at high levels, known as CXCL12-abundant reticular (CAR) cells,22,23 to study the localization of pDCs within bone marrow during development. Immunohistochemical analysis using PDCA-1 was performed to visualize pDCs, since pDCs could be identified as B220+PDCA-1+ cells,27 and almost all PDCA-1+ cells were B220+ in the bone marrow sections (data not shown). Analysis of bone marrow from mice with the GFP reporter gene knocked into the CXCL12 locus (CXCL12/GFP knock-in mice)21,–23 revealed that PDCA-1+ pDCs were found scattered throughout the marrow cavity, and most PDCA-1+ pDCs were in contact with CAR cells (340 [89%] of 382; Figure 5A,B). Immunohistochemical analysis of CXCL12/GFP knock-in mice using antibodies against Sca-1 and c-kit revealed that most Sca-1+c-kit+ primitive hematopoietic cells, including HSCs, were in contact with CAR cells,22 and around half of Sca-1+c-kit+ primitive hematopoietic cells adjoined CAR cells that surrounded PECAM-1+ sinusoidal endothelial cells (17 [52%] of 33). In addition, some remaining Sca-1+c-kit+ primitive hematopoietic cells adjoined CAR cells that resided near the bone surface (7 [21%] of 33). In contrast to Sca-1+c-kit+ primitive hematopoietic cells, only a small number of PDCA-1+ pDCs adjoined CAR cells that were associated with morphologically identifiable sinusoidal endothelial cells (39 [11%] of 340) or that resided near the bone surface (13 [3.8%] of 340; Figure 5A,B).
Association of pDCs with cellular niches in the bone marrow of wild-type and CXCR4-deficient mice. (A,B) Bone marrow sections from CXCL12/GFP knock-in mice stained with antibodies against PDCA-1 (red) and PECAM-1 (white) (A). Many PDCA-1+ pDCs (arrows; red) are found in close association with CAR cells (green) in the intersinal space (A). S indicates vascular sinus. Scale bars equal 10 μm. (B) Frequency of PDCA-1+ pDCs in contact with CAR cells and CAR cells surrounding morphologically identifiable PECAM-1+ sinusoidal endothelial cells or residing near the bone surface. (C) Flow cytometric analysis of the numbers of LSK Flt3lo primitive hematopoietic cells and CD11cintB220+PDCA-1+ pDCs in bone marrow of pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt mice, untreated CXCL12/GFP/MxCre/CXCR4f/null mice, and pIpC-treated CXCL12/GFP/MxCre/CXCR4f/null mice 1 week after the final pIpC treatment (n = 3). (D) Bone marrow sections from pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt (left) and CXCL12/GFP/MxCre/CXCR4f/null mice (right) stained with antibodies against PDCA-1 (red). Many PDCA-1+ pDCs (arrows; red) are found in close association with CAR cells (green) in pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt mice (left), but PDCA-1+ pDCs are undetectable in pIpC-treated CXCL12/GFP/MxCre/CXCR4f/null mice (right). Scale bars equal 10 μm.
Association of pDCs with cellular niches in the bone marrow of wild-type and CXCR4-deficient mice. (A,B) Bone marrow sections from CXCL12/GFP knock-in mice stained with antibodies against PDCA-1 (red) and PECAM-1 (white) (A). Many PDCA-1+ pDCs (arrows; red) are found in close association with CAR cells (green) in the intersinal space (A). S indicates vascular sinus. Scale bars equal 10 μm. (B) Frequency of PDCA-1+ pDCs in contact with CAR cells and CAR cells surrounding morphologically identifiable PECAM-1+ sinusoidal endothelial cells or residing near the bone surface. (C) Flow cytometric analysis of the numbers of LSK Flt3lo primitive hematopoietic cells and CD11cintB220+PDCA-1+ pDCs in bone marrow of pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt mice, untreated CXCL12/GFP/MxCre/CXCR4f/null mice, and pIpC-treated CXCL12/GFP/MxCre/CXCR4f/null mice 1 week after the final pIpC treatment (n = 3). (D) Bone marrow sections from pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt (left) and CXCL12/GFP/MxCre/CXCR4f/null mice (right) stained with antibodies against PDCA-1 (red). Many PDCA-1+ pDCs (arrows; red) are found in close association with CAR cells (green) in pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt mice (left), but PDCA-1+ pDCs are undetectable in pIpC-treated CXCL12/GFP/MxCre/CXCR4f/null mice (right). Scale bars equal 10 μm.
We next analyzed the interaction of pDCs with CAR cells in the absence of CXCR4 using CXCR4 conditionally deficient mice. We crossed MxCre/CXCR4f/null mice with CXCL12/GFP knock-in mice.22 Mice were then treated with pIpC to induce excision of the floxed allele and were analyzed 1 week after the final pIpC treatment. Flow cytometric analysis revealed that the numbers of Lin−Sca-1+c-kit+Flt3lo primitive hematopoietic cells appeared normal, but the numbers of B220+PDCA-1+ pDCs were severely reduced in pIpC-treated CXCL12/GFP/MxCre/CXCR4f/null mice compared with untreated CXCL12/GFP/MxCre/CXCR4f/null mice and control pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt mice (Figure 5C). Immunohistochemical analysis revealed that most PDCA-1+ pDCs were found in contact with CAR cells in pIpC-treated CXCL12/GFP/MxCre/CXCR4f/wt mice, but PDCA-1+ pDCs were undetectable in the bone marrow of pIpC-treated CXCL12/GFP/MxCre/CXCR4f/null mice, indicating that CXCR4 is essential for the generation of pDCs, which reside in proximity to CAR cells (Figure 5D).
Discussion
We have shown that CXCL12-CXCR4 chemokine signaling is essential for the development of pDCs. Lin−Sca-1+c-kit+Flt3lo primitive hematopoietic cells were modestly reduced, but pDC progenitors, including cells in Flt3+ subsets of CLP and CMP fractions as well as pDCs, were severely reduced in the absence of CXCR4. In addition, CXCL12 induced a significant increase in the numbers of pDCs generated from primitive hematopoietic cells, but had little effect on the generation of pDCs from pDC progenitors, including cells in Flt3+ subsets of CLP and CMP fractions in the presence of Flt3L in vitro. These results suggest that CXCL12 acts on primitive hematopoietic cells, enhancing the generation of early pDC precursors. Because pDCs could be produced from primitive hematopoietic cells even in the absence of CXCL12 in vitro, and primitive hematopoietic cells, pDC progenitors, and pDCs migrated to CXCL12 in chemotaxis assays, it raises the possibility that CXCL12 also plays a role in attracting and/or tethering pDC progenitors and pDCs in their specific niches in which essential factors, including Flt3L and CXCL12, act on these cells in vivo. Considering the results that most primitive hematopoietic cells22,23 and pDCs were in contact with CAR cells, CAR cells are candidates for the niches for pDC development within bone marrow. It has been reported previously that many HSCs reside near the bone marrow sinusoidal endothelial cells, and some HSCs adjoin the endosteum.36 Consistent with this, the majority of the primitive hematopoietic cells, including HSCs were in contact with CAR cells surrounding sinusoidal endothelial cells or residing near the bone surface. In contrast, only a small number of PDCA-1+ pDCs adjoined CAR cells that were associated with endothelial cells or that resided near the bone surface, suggesting that cells after commitment to pDC lineage move away from vascular and/or endosteal niches to the intersinal spaces.
On the other hand, the development of pDCs and B cells are severely impaired relative to other lineages, including myeloid cells, T cells, and cDCs in the absence of CXCL12-CXCR4 signaling.20,22,24 This result raises the possibility that bipotential progenitors of pDCs and B cells exist, and that CXCL12 acts on presumptive progenitors. Further characterization of the pathway of early pDC lineage differentiation will be needed.
Previous studies have shown that there are functional associations between CXCL12 and Flt3L in the migration, survival, and proliferation of hematopoietic cells.37,38 It is now clear that both CXCL12 and Flt3L are essential for pDC development, and the deficits in the development of pDCs and cDCs in the absence of CXCR4 are similar to the defects observed in Flt3L-deficient mice. However, in vitro experiments revealed that Flt3L but not CXCL12 acted on pDC progenitors, including cells in Flt3+ subsets of CLP and CMP fractions in the production of pDCs, raising the possibility that CXCL12 might play a specific role in earlier stages compared with Flt3L in pDC development. On the other hand, in contrast to pDCs, phenotypic differences between mutants lacking Flt3L-Flt3 and CXCL12-CXCR4 signaling exist in B-cell development. Flt3L-deficient mice display deficits in generation of pro-B, pre-B, immature, and mature B cells that are much less severe than those seen in the absence of CXCR4,8,20,22 indicating that CXCL12 dependency is much stronger than Flt3L dependency in B-cell development. It is important to know how interaction between these 2 cytokines influence fate of pDCs in bone marrow.
In addition to an antimicrobial immune response, it has been suggested that pDCs activated by autoantibody-DNA complexes contribute to the pathogenesis of autoimmune diseases, including systemic lupus erythematosus (SLE).39 Therefore, the pDC population will likely have therapeutic applications if its proliferation and differentiation can be controlled. On the other hand, a specific antagonist of CXCL12-CXCR4 signaling is currently used in the clinic for HSC transplantation.40,–42 Together, this study provides a novel basis to understand the environmental regulation of pDC development and provides new targets for its therapeutic control.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank K. Rajewsky and K. Kitamura for providing MxCre mice, and T. Egawa, S. Mikami, and M. Sato for technical assistance.
This work was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan.
Authorship
Contribution: H.K., Y.O., and T.N. designed and performed the research, analyzed data, and wrote the paper; T.S. designed and performed the research; M.N. performed the research; N.F. contributed vital reagents; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takashi Nagasawa, Department of Immunobiology and Hematology, Institute for Frontier Medical Sciences, Kyoto University, 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan; e-mail:tnagasa@frontier.kyoto-u.ac.jp.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal