LSCs share many of the inherent properties of normal hematopoietic stem cells (HSCs), making the selective targeting of LSCs a significant challenge for investigators. Guzman et al addressed this challenge using a variety of approaches that have already been productive, and they collectively demonstrate that it may indeed be possible to target the LSC population without necessarily causing damage to normal HSCs.1,–3
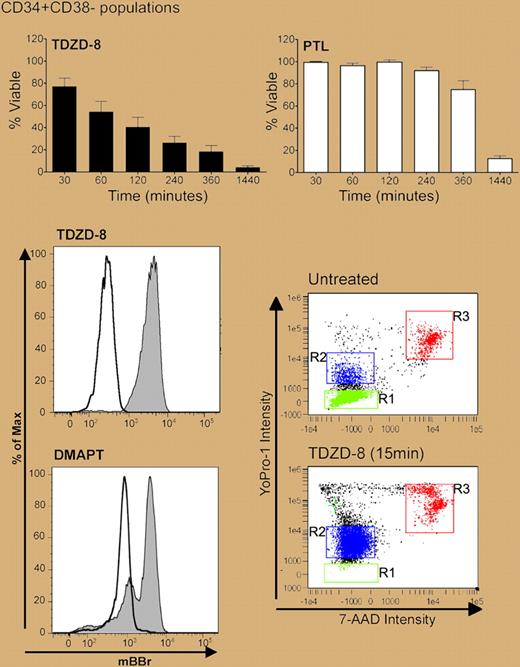
In a search for inhibitors of pathways implicated in early hematopoiesis, the authors investigated a large number of GSK-3β inhibitors and found that TDZD-8 was potently cytotoxic to multiple primary leukemic cells, including AML, ALL, CLL, and blast-crisis CML. The drug also killed phenotypically and functionally defined LSCs, with little bystander effect on normal HSCs. Although not fully understood, the mechanism of action involved very rapid loss of membrane integrity and irreversible cell damage within 15 to 30 minutes (see figure). As shown in their previous work,2,3 the authors found that TDZD-8 exposure also led to inhibition of NF-κB and induction of oxidative stress, both of which the authors argue are critical components of LSC-selective killing.
Rapid effect of TDZD-8 compared with PTL on CD34+38− AML cell viability (top panel). Loss of membrane integrity as measured by MBBr with both TDZD-8 and DMAPT (bottom left; shaded histograms) and YoPro-1 (bottom right; TDZD-8). See the complete figures in the articles beginning on pages 4427 and 4436.
Rapid effect of TDZD-8 compared with PTL on CD34+38− AML cell viability (top panel). Loss of membrane integrity as measured by MBBr with both TDZD-8 and DMAPT (bottom left; shaded histograms) and YoPro-1 (bottom right; TDZD-8). See the complete figures in the articles beginning on pages 4427 and 4436.
This new work is exciting because it suggests that there are potent agents that can reach into the LSC compartment. Potential limitations of the work include the high concentration of TDZD-8 that was required to induce LSC apoptosis and the fact that its cytotoxic effect was mediated not through inhibition of GSK-3β, but by an “off-target” effect. While this is scientifically intriguing, it creates a major challenge for developing an analog that can be used clinically, since the true mechanism of action of TDZD-8 remains elusive. It is also difficult to understand, from the work presented to date, how this cytotoxic agent achieves its apparent level of selectivity for LSCs versus HSCs.
In the companion paper, the authors confirm that DMAPT has equal potency to PTL in inducing selective death of LSCs from both myeloid and lymphoid leukemias. Although PTL and DMAPT inhibit NF-κB, induce oxidative stress responses, and activate p53, the kinetics of response are much slower that those of TDZD-8 (see figure). Preliminary evidence for in vivo bioactivity was demonstrated through murine xenograft models and against a few cases of spontaneous canine leukemia. Although tantalizing, these preclinical canine data should be interpreted with caution, since few animals were included and all received potentially confounding drugs. We eagerly await the phase 1 clinical trial data.
Overall, Guzman and colleagues are to be congratulated for their perse-verance, which has now delivered new and important weapons against the Achilles heel of hematological malignancy, the LSC.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal