Abstract
Interpatient variability in intracellular uptake and retention (IUR) of imatinib may be due to variable function of the OCT-1 influx pump. OCT-1 activity was measured in pretherapy blood from chronic myeloid leukemia (CML) patients by calculating the difference in IUR of [14C]-imatinib with and without OCT-1 inhibition. Of patients with higher than median (high) OCT-1 activity, 85% achieved major molecular response (MMR) by 24 months, versus 45% with no more than a median (low) OCT-1 activity. Assessing patients receiving 600 mg imatinib per day and those averaging fewer than 600 mg over 12 months of therapy revealed patients with high OCT-1 activity achieved excellent molecular response regardless of dose, whereas response of patients with low OCT-1 activity was highly dose dependent. Of patients with low OCT-1 activity who received fewer than 600 mg, 45% failed to achieve a 2-log reduction by 12 months, and 82% failed to achieve a MMR by 18 months, compared with 8% and 17% in the cohort with high OCT-1 activity and dose less than 600 mg/day (P = .017 and P = .022). OCT-1 activity is an important determinant of molecular response to imatinib, with predictive value closely linked to dose. This pretherapy assay identifies patients at greatest risk of suboptimal response where dose intensity is critical, and those likely to respond equally well to standard dose imatinib.
Introduction
Imatinib mesylate (Glivec, Novartis Pharmaceuticals) is a 2-phenylaminopyramidine derivative that potently inhibits the ABL tyrosine kinase activity of c-ABL, BCR-ABL, and Tel-ABL. The treatment of chronic myeloid leukemia (CML) with imatinib has met significant success, in terms of both prolonged survival and improved quality of life.1-3
While overall responses to standard dose imatinib therapy have been excellent,4 the poorer response observed in a small proportion of patients raises the possibility that higher doses may be beneficial in these cases. While a strategy of dose escalation has proven valuable in some patients5 to understand why some, but not all, patients respond well to standard dose, and to identify those patients likely to benefit most with higher doses of imatinib or second generation kinase inhibitor therapy, reliable predictive assays based on a sound scientific rationale are required.
Using the phosphorylation status of the adaptor protein Crkl, an immediate downstream partner of BCR-ABL, we have demonstrated that previously untreated, newly diagnosed CML patients have differing intrinsic sensitivities to BCR-ABL tyrosine kinase inhibition.6 Furthermore, intrinsic sensitivity was shown to be predictive of molecular response in the setting of the Trial of Imatinib with Dose Escalation in chronic myeloid Leukemia (TIDEL). Thomas et al7 suggested that the uptake of imatinib into target hemopoietic cells is dependent on the human organic cation transporter-1 (OCT-1). We subsequently demonstrated, using 14C-labeled imatinib, that there was a significant difference in drug uptake between patients with low- and high-intrinsic sensitivity, and importantly, that functional inhibition of the OCT-1 protein removed this difference.8 It also has been demonstrated that the level of OCT-1 mRNA is significantly lower in cytogenetic nonresponders, but this is yet to be confirmed in a de novo cohort.9,10
The OCT-1 protein is a member of the largest superfamily of transporters, the solute carrier family,11 which transport in an electrogenic fashion a variety of organic cations including drugs, toxins, and other xenobiotics. The transporter is predicted to have 12 transmembrane domains and binding pockets with partially overlapping interaction domains for different substrates and inhibitors.12 Polymorphisms that affect function have been reported.13,14 Post-transcriptional regulation of OCT-1 by phosphorylation status15 and such compounds as protein kinase A (PKA), Src-like p56, and CaM also have been demonstrated.16-18
Both quantitative and qualitative changes in OCT-1 may have an impact on imatinib uptake. We hypothesized that a functional assay of imatinib uptake into patient cells may provide the most clinically predictive assay of OCT-1 function. To determine the functional level of the OCT-1 protein, we have used prazosin, a potent inhibitor of OCT-1 (IC50 value, 1.8 μM) and, to a lesser extent, OCT-3 (IC50 value 13 μM), but not OCT-219 to inhibit OCT-1 from transporting imatinib into the cell. We reasoned that the difference between the total intracellular uptake and retention (IUR) of imatinib, determined, using [14C]-labeled imatinib, in the presence and in the absence of prazosin, will provide an estimate of the functional activity of the OCT-1 protein with respect to imatinib transport. We term this the imatinib OCT-1 activity. We have then compared this OCT-1 activity with the IUR of imatinib and the IC50imatinib. Furthermore, by linking this data to a clinical trial, we assess the predictive value of OCT-1 activity with respect to molecular response and explore the relationship between OCT-1 activity and differential dose response. These studies provide valuable information on the role of the OCT-1 protein in achieving an adequate intracellular concentration of imatinib and give further insight into the variable responses seen in some CML patients treated with imatinib. Importantly, this knowledge may in the future be used to develop individualized patient treatment strategies, aimed at delivering maximal and early molecular responses.
Patients, materials, and methods
Patient samples
Patients in this study were enrolled in TIDEL and the Tyrosine kinase inhibitor Optimization and Selectivity (TOPS) trial. TIDEL was a phase 2 study in adult patients with newly diagnosed CML. All patients commenced imatinib at 600 mg/day. The dose was increased to 800 mg/day if a patient failed to achieve any of the following: (1) complete hematological response (CHR) by 3 months, (2); MCyR by 6 months, (3) CCyR by 9 months, or (4) 4-log reduction in BCR-ABL from the standardized baseline by 12 months. TOPS is an ongoing trial of newly diagnosed adult CML patients randomized to either 400 mg or 800 mg imatinib per day. Data from TOPS patients was used only to establish relationships between IC50, IUR, OCT-1 activity, and mRNA levels. No molecular data from TOPS was included in these analyses.
Sixty millileters of blood was obtained from newly diagnosed, chronic phase CML patients prior to the commencement of imatinib therapy. For all analyses mononuclear cells (PBMNCs) were isolated using Lymphoprep (Axis Shield, Oslo, Norway) density gradient centrifugation.6 All patients enrolled were within 9 months of diagnosis and had received only hydroxyurea therapy prior to enrolment. Blood samples were collected and informed consent was obtained in accordance with the Declaration of Helsinki.
Imatinib mesylate
The 2-phenylaminopyrimidine derivatives imatinib mesylate (Gleevec, formerly STI571), together with [14C]-STI571, were kindly provided by Novartis Pharmaceuticals (Basel, Switzerland).
Western blot analysis and determination of IC50 values
Western blot assays for p-Crkl were performed essentially as previously described.6 Briefly, patient cells were incubated for 2 hours at 37°C with concentrations of imatinib ranging from 0 μM to 50 μM. Following incubation, cells were washed once with cold phosphate-buffered saline (PBS) and lysed in Laemmli buffer20 by boiling for 12 minutes. Lysates were clarified by microfugation and stored at −20°C. Protein lysates were resolved on a sodium dodecyl sulfate (SDS)/10% polyacrylamide gel and electrophoretically transferred to polyvinylidenefluoride (PVDF) (Amersham Biosciences, Giles, United Kingdom). Following blocking, the membrane was probed with anti-Crkl antibody (Santa Cruz Biotechnology, Santa Cruz, CA), detected with ECF substrate (Amersham Pharmacia, Piscataway, NJ) and analyzed by Fluor Imager analysis (Molecular Dynamics. Sunnyvale, CA). Signals were quantified using Image Quant software (Molecular Dynamics), and the ratio of phosphorylated Crkl (p-Crkl) to nonphosphorylated Crkl was determined using Image Quant analysis. IC50 values were determined as the dose of drug required to reduce phosphorylation of the adaptor protein Crkl (p-Crkl) by 50%. High and low IC50imatinib groups were dichotomized on the median IC50imatinib value.
Radiolabeled drug uptake (IUR assay)
The IUR assay was performed as previously described.8 In brief, 200 000 PBMNCs were incubated for 2 hours at 37°C in the presence of varying concentrations of imatinib ranging from 0 μM to 2 μM. Isotopes were resuspended to 1 mg/mL the specific activity of [14C]-STI571 was 3.3 MBq. After incubation the cellular and aqueous phases were separated and incorporation determined using a Top Count Microplate Beta Scintillation counter (Perkin Elmer, Boston, MA) following the addition of Microscint20 scintillation fluid (Perkin Elmer). All assays were performed in triplicate and repeated if the assay demonstrated nonconcordance. The IUR was analyzed in K562 cells as a control for reproducibility in all assays. Where the K562 assays fell outside of the 95% confidence limits, the assay results were not included and the assay was repeated.
OCT-1 activity
The OCT-1 activity was determined using the potent OCT-1 inhibitor Prazosin.19 Assays were performed in the presence and absence of Prazosin at 100 μM. The OCT-1 activity is described as the difference between the IUR in the absence of Prazosin and the IUR in the presence of Prazosin. Confirmation that the difference in intracellular uptake of imatinib with and without Prazosin was predominantly due to the inhibition of OCT-1 was made by parallel assays using Procainamide to inhibit OCT-1 in 59 patients. Procainamide at a concentration of 100 μM inhibits OCT-1 and OCT-2, but not OCT-3, whereas Prazosin at 100 μM inhibits OCT-1 and OCT-3.12,21,22
OCT-1 mRNA levels
RNA was extracted from a minimum of 5 × 106 cells using TRIzol Reagent (Invitrogen, Carlsbad, CA). Complementary DNA (cDNA) was synthesized using Random Hexamers (GeneWorks, Hindmarsh, Australia) and Superscript II Reverse Transcriptase (Invitrogen). Primers were designed using Primer Express software v2.0 (Applied Biosystems, Foster City, CA), and sequences were as follows:- hOCT-1 F CTG AGC TGT ACC CCA CAT TCG: hOCT-1 R CCA ACA CCG CAA ACA AAA TGA (Sigma-Aldrich, Castle Hill, Australia). Amplification was performed using RT2 real-time SYBR Green/ROX PCR Master Mix (SuperArray Bioscience, Frederick, MD) on a RotorGene real time polymerase chain reaction (PCR) machine (Corbett Biosciences, San Francisco, CA).
Response criteria used for analysis
The molecular response as determined by real-time quantitative reverse transcriptase PCR (RQ-PCR) for BCR-ABL was used to monitor response and compare outcomes between different groups. This analysis was performed only on the TIDEL patients in this study. An optimal molecular response was defined as the achievement of major molecular response (MMR:3-log reduction from standardized baseline in BCR-ABL) as measured by quantitative reverse transcriptase PCR (RQ-PCR) by 12 and 24 months. The achievement of a 4-log reduction in BCR-ABL by 12 and 24 months also was assessed.
Statistics
All statistical analyses were performed using Sigma Stat Software. The t test was used to define differences between groups, and correlation was performed using the Pearson product moment. Kaplan-Meier curves were constructed to estimate the probability of achievement of log reductions in BCR-ABL, and log-rank survival analysis provided comparative statistics. To assess the difference between groups, Kruskal-Wallis one-way analysis of variance on ranks was performed.
Only data from patients enrolled in TIDEL were used for correlation with molecular response, as TOPS is an ongoing trial and molecular data has not yet been analyzed. TOPS data were used for correlations between baseline assays only.
Results
Correlation between IC50imatinib and IUR of [14C]-imatinib
In a smaller series (n = 19), we previously demonstrated a good correlation between the IC50imatinib and the IUR (P = .014).8 In the present communication, we extend this finding using an expanded series (n = 99) and confirm the correlation (r = -.342; P = .005), between IC50imatinib and the IUR (Figure 1A). Furthermore, grouping IC50imatinib into low and high about the median, we show a significant difference between the IUR of the low and high IC50imatinib groups (P = .001), but that this difference is removed when the OCT-1 inhibitor Prazosin is added (P = .129) (Figure 1B). This again confirms our previous findings8 and those of others7 and demonstrates the importance of the transporter OCT-1 in imatinib influx.
Correlation between IC50imatinib and the IUR of imatinib, and IC50 and OCT-1 activity. (A) Showing the correlation between the IC50imatinib and the IUR in 99 patients. (B) Box plot demonstrating the difference in IUR between low and high IC50imatinib groups, and also demonstrating the removal of this difference using prazosin. (C) Showing the correlation between the IC50imatinib and the OCT-1 activity in 99 patients. (D) Box plot demonstrating the difference in OCT-1 activity between low and high IC50imatinib groups.
Correlation between IC50imatinib and the IUR of imatinib, and IC50 and OCT-1 activity. (A) Showing the correlation between the IC50imatinib and the IUR in 99 patients. (B) Box plot demonstrating the difference in IUR between low and high IC50imatinib groups, and also demonstrating the removal of this difference using prazosin. (C) Showing the correlation between the IC50imatinib and the OCT-1 activity in 99 patients. (D) Box plot demonstrating the difference in OCT-1 activity between low and high IC50imatinib groups.
OCT-1 activity
The addition of prazosin, a potent inhibitor of OCT-1, to the IUR assay impairs the active transport of imatinib by OCT-1. We calculated OCT-1 activity as the difference in IUR in the absence (total IUR) and presence of prazosin, to provide a measure of the actual activity of the OCT-1 protein in the transport of imatinib. For example: [total IUR 32 ng/200 000 cells)]—[prazosin IUR 23 ng/200 000 cells] gives an OCT-1 activity of 9 ng/200 000 cells. In 59 patients, we confirmed inhibition of OCT-1 using procainamide and show a correlation between the OCT-1 activities using the 2 inhibitors to be R = 0.945; P < .001 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Furthermore, we also show the Chi square analysis when OCT-1 activities were dichotomized about the median for each inhibitor, to be 31.007 (P < .001).
Examination of 132 patients enrolled in both trials reveals a wide variation in OCT-1 activity (median, 8.2; range, 0 to 31.2). In replicate assays of 5 patients, the IUR values with prazosin were equal or higher than the values without prazosin. We scored these patients as having negligible (0 ng/200 000 cells) OCT-1 activity.
OCT-1 activity and IC50imatinib
In 99 patients where both IC50imatinib and OCT-1 activity were measured, there was a correlation between the IC50imatinib and the OCT-1 activity (r = -.238; P = .019) (Figure 1C). In addition, grouping IC50imatinib into low and high according to the median value revealed a significantly greater OCT-1 activity in the low IC50imatinib group when compared with the high group (P = .008) (Figure 1D), suggesting that low IC50imatinib and, therefore, drug effect is strongly related to intracellular uptake.
OCT-1 activity and Sokal prognostic score
The OCT-1 activity does not correlate with the Sokal prognostic score23 (r = 0.019; P = .890), and there is no significant difference between the median Sokal scores of the low and high OCT-1 activity groups (low OCT-1 activity 0.96; high OCT-1 activity 0.975; P = .712).
OCT-1 activity, molecular response, and the effect of actual dose received
The OCT-1 activity was compared with molecular response over the first 24 months of imatinib therapy in 56 patients enrolled in the TIDEL trial and for whom samples were available for this analysis. Patients were grouped into low- and high-OCT-1 activity based on the median activity (7.2 ng/200 000 cells) for this cohort, as shown in Table 1. Patients with high OCT-1 activity (n = 27) achieved significantly higher molecular response over the time course than patients with low OCT-1 activity (n = 29; P = .005 at 24 months).
OCT-1 activity and molecular response
| Months after treatment . | Average molecular response at 6-month intervals . | |||
|---|---|---|---|---|
| 12 . | 18 . | 24 . | ||
| OCT-1 activity | ||||
| Low (n=29) | 2.6 | 2.6 | 2.8 | |
| High (n=27) | 3.1 | 3.9 | 3.9 | |
| P value | .032 | .006 | .005 | |
| Low OCT-1 activity | ||||
| Less than 600 mg (n=11) | 2.1 | 2.3 | 2.4 | |
| 600 mg or more (n=18) | 2.8 | 3.2 | 3.4 | |
| P value | .121 | .023 | .005 | |
| High OCT-1 activity | ||||
| Less than 600 mg (n=12) | 2.9 | 3.3 | 3.5 | |
| 600 mg or more (n=15) | 2.9 | 3.9 | 3.9 | |
| P value | .789 | .625 | .449 | |
| Months after treatment . | Average molecular response at 6-month intervals . | |||
|---|---|---|---|---|
| 12 . | 18 . | 24 . | ||
| OCT-1 activity | ||||
| Low (n=29) | 2.6 | 2.6 | 2.8 | |
| High (n=27) | 3.1 | 3.9 | 3.9 | |
| P value | .032 | .006 | .005 | |
| Low OCT-1 activity | ||||
| Less than 600 mg (n=11) | 2.1 | 2.3 | 2.4 | |
| 600 mg or more (n=18) | 2.8 | 3.2 | 3.4 | |
| P value | .121 | .023 | .005 | |
| High OCT-1 activity | ||||
| Less than 600 mg (n=12) | 2.9 | 3.3 | 3.5 | |
| 600 mg or more (n=15) | 2.9 | 3.9 | 3.9 | |
| P value | .789 | .625 | .449 | |
Shown is the molecular response (log reduction in BCR-ABL as measured by real-time quantitative polymerase chain reaction [RQ-PCR]) of patients enrolled in TIDEL at 6-month intervals based on low and high OCT-1 activity, then further on the basis of dose. Data are log reductions except for P values.
However, because of tolerability issues, not all patients received 600 mg of imatinib consistently over the first 12 months of therapy. To assess the effect of varying dose, patients were further subgrouped into those patients who received an average daily dose (ADD) of 600 mg (n = 33) over the first 12 months of imatinib therapy, and those who received an ADD of less than 600 mg per day (n = 23 median ADD 523 mg). Four patients with ADD lower than 400 mg were included in this cohort.
Assessing only those patients with high OCT-1 activity, there is no significant difference in molecular response between those patients who received less than 600 mg versus those patients who received 600 mg or more over the first 12 months (P = .449 at 24 months) (Table 1). In contrast, in the cohort of patients with low OCT-1 activity, there is a significant dose effect, with patients receiving 600 mg achieving significantly better molecular responses than those who fail to receive 600 mg (P = .005 at 24 months) (Table 1).
Kaplan-Meier analysis revealed an 85% probability of achieving MMR by 24 months in patients with high OCT-1 activity (median time to MMR, 9 months) versus only 45% of patients with low OCT-1 activity (median time, 24 months) P = .009 (Figure 2). Analyzing only those patients who received less than 600 mg ADD over the first 12 months reveals a significant effect of dose between low- and high-OCT-1 activity patients. Of those patients with high OCT-1 activity (n = 12), 83% achieve MMR compared with only 18% of patients with a low OCT-1 activity (n = 11; P = .022). Among the 33 patients receiving an ADD of 600 mg, there was no significant difference according to OCT-1 activity (P = .110).
Kaplan-Meier demonstrating the difference in achievement of MMR by 24 months between low and high OCT-1 activity groups.
Kaplan-Meier demonstrating the difference in achievement of MMR by 24 months between low and high OCT-1 activity groups.
Performance of a 2-way analysis of variance, with the dependent variable being molecular response at 24 months and dichotomizing OCT-1 activity into low- and high-based on median values, and ADD to 12 months into 600 mg or not, we show that the effect of different levels of dose to 12 months depends on the level of OCT-1 activity (P = .018). This demonstrates a significant interaction between OCT-1 activity and dose in the determination of 24-month molecular response.
Effect of dose increase
In the TIDEL trial, dose increases from the initial 600 mg to 800 mg per day were mandated if a 4-log reduction in BCR-ABL from the standardized baseline was not achieved by 12 months. In our patient cohort, 46 patients were scheduled to receive dose increases. In this analysis, a dose increase was considered to have occurred if the patient received 800 mg ADD for at least one month.
For reasons of toxicity, only 29 of 46 (63%) patients in this cohort successfully dose increased to 800 mg per day. The primary reason for inability to increase dose was previous toxicity/tolerance issues, occurring in 13 of those 17 patients who did not dose escalate. Of the remaining 4 patients, 2 dose escalated for one month but did not reach 600 mg ADD. It is not known why the remaining 2 patients failed to escalate.
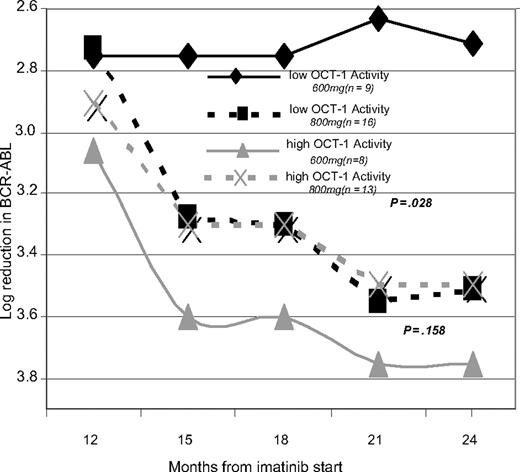
Dose increase occurred by 14 months in all patients who were able to tolerate dose increase. To assess the impact of dose escalation, patients were grouped into low- and high-OCT-1 activity groups as previously, then were further subdivided into those who received increased dose and those who remained on 600 mg or less as shown in Figure 3. In the high-OCT-1 activity cohort, there was no significant difference in molecular response between the patients who dose increased and the patients who failed to dose increase (P > .05 at all time points). In contrast, patients with a low OCT-1 activity performed equally well as those patients with high activity when they are dose increased. However, those who failed to dose increase in the presence of low OCT-1 activity had significantly inferior molecular responses at 24 months (Figure 3).
Demonstrating the effect of dose escalation by dividing patients in to low and high OCT-1 activity groups and then further subdividing them on the basis of ADD received after 12 months.
Demonstrating the effect of dose escalation by dividing patients in to low and high OCT-1 activity groups and then further subdividing them on the basis of ADD received after 12 months.
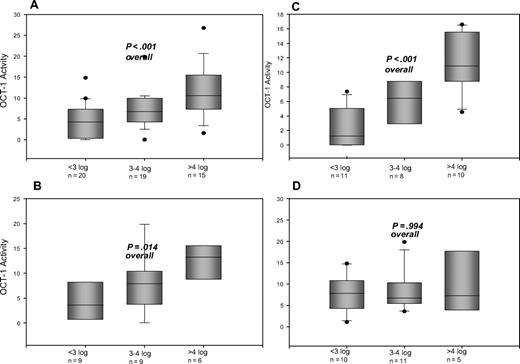
The median OCT-1 activity in patients who failed to achieve MMR by 18 months is significantly lower than OCT-1 activity in patients who did achieve MMR (Figure 4). These differences in OCT-1 activity between molecular response groups is most pronounced in patients who were not able to dose escalate at 12 months (Figure 4C). In patients who dose increased at 12 months, there is no significant difference between the 3 groups (Figure 4D).
The OCT-1 activity compared with molecular responses (log reduction in BCR-ABL) at 18 months. Response criteria assessed are suboptimal response (failure to achieve MMR by 18 months) and optimal responses (achievement of 3- to 4-log reduction in BCR-ABL, and more than 4-log reduction by 18 months. Cohorts are divided: (A) All patients irrespective of dose received. (B) Patients receiving less than 600 mg ADD over the first 12 months. (C) Patients who failed to dose escalate to 800 mg ADD after 12 months. (D) Patients who successfully dose escalated to 800 mg ADD after 12 months of therapy.
The OCT-1 activity compared with molecular responses (log reduction in BCR-ABL) at 18 months. Response criteria assessed are suboptimal response (failure to achieve MMR by 18 months) and optimal responses (achievement of 3- to 4-log reduction in BCR-ABL, and more than 4-log reduction by 18 months. Cohorts are divided: (A) All patients irrespective of dose received. (B) Patients receiving less than 600 mg ADD over the first 12 months. (C) Patients who failed to dose escalate to 800 mg ADD after 12 months. (D) Patients who successfully dose escalated to 800 mg ADD after 12 months of therapy.
OCT-1 activity, suboptimal response, and imatinib failure
Suboptimal response has been defined as failure to achieve a major cytogenetic response by 6 months, a complete cytogenetic response by 12 months, or an MMR by 18 months.24 As good correlation between molecular and cytogenetic responses has been demonstrated previously,25 we assessed the suboptimal response as failure to achieve 1-log reduction in BCR-ABL by 6 months, a 2-log reduction by 12 months, and a 3-log reduction (MMR) by 18 months. Log-rank survival analysis as reported in Table 2 reveals there is no significant difference in the frequency of suboptimal response at any timepoint when patients receive 600 mg ADD; however, there is a significant difference at all timepoints when patients receive reduced dose, demonstrating patients with low OCT-1 activity who receive reduced dosing (median ADD for this cohort, 511 mg) are at substantial risk of suboptimal response to imatinib.
Suboptimal response to imatinib therapy
| . | Percentage of patients failing to achieve reductions . | ||
|---|---|---|---|
| 1-log reduction in BCR-ABL by 6 months . | 2-log reduction in BCR-ABL by 12 months . | 3-log reduction in BCR-ABL by 18 months . | |
| Patients receiving 600 mg or more ADD | |||
| High OCT-1 activity (n = 15) | 7 | 13 | 20 |
| Low OCT-1 activity (n = 18) | 0 | 0 | 44 |
| P value | .669 | .560 | .133 |
| Patients receiving less than 600 mg | |||
| High OCT-1 activity (n = 12) | 0 | 8 | 17 |
| Low OCT-1 activity (n = 11) | 27 | 45 | 82 |
| P value | .005 | .021 | .022 |
| . | Percentage of patients failing to achieve reductions . | ||
|---|---|---|---|
| 1-log reduction in BCR-ABL by 6 months . | 2-log reduction in BCR-ABL by 12 months . | 3-log reduction in BCR-ABL by 18 months . | |
| Patients receiving 600 mg or more ADD | |||
| High OCT-1 activity (n = 15) | 7 | 13 | 20 |
| Low OCT-1 activity (n = 18) | 0 | 0 | 44 |
| P value | .669 | .560 | .133 |
| Patients receiving less than 600 mg | |||
| High OCT-1 activity (n = 12) | 0 | 8 | 17 |
| Low OCT-1 activity (n = 11) | 27 | 45 | 82 |
| P value | .005 | .021 | .022 |
Data are percentages except for P values.
Assessing the failure to achieve a 2-log reduction by 18 months (imatinib failure24 ) reveals also that a significantly lower proportion of patients with low OCT-1 activity who receive reduced dose achieve a 2-log reduction by 18 months (low OCT-1 activity 36% versus high OCT-1 activity 8%, P = .04).
OCT-1 mRNA and OCT-1 activity
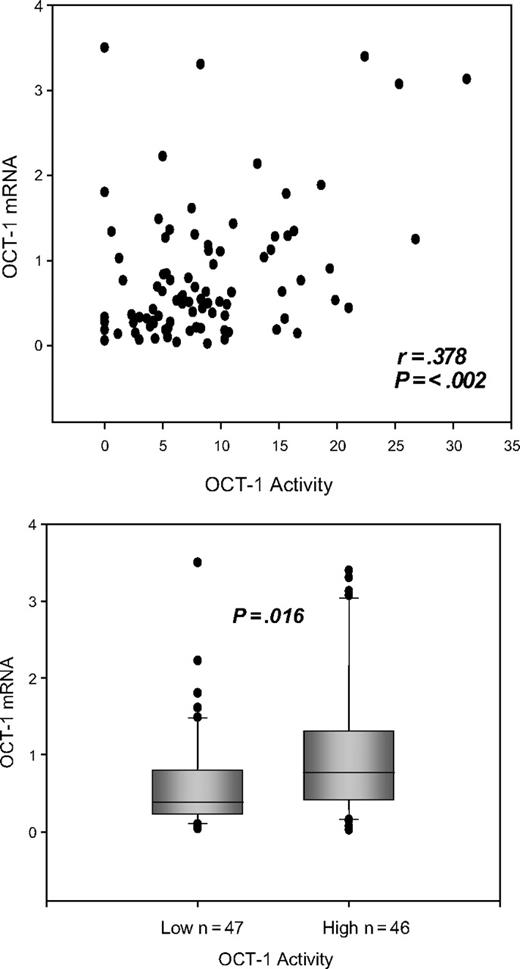
The level of OCT-1 mRNA was measured relative to BCR mRNA in 93 patients. The relative mean percent OCT-1/BCR mRNA was 0.835 within a range of 0.02% to 3.5%. Measures of OCT-1 mRNA were closely correlated with OCT-1 activity (P = .002; r = 0.378 Pearson product moment) (Figure 5). Dividing patients into low- and high-OCT-1 activity about the median for this cohort of 7.9 reveals a significant difference between the 2 groups. The median percent OCT-1 mRNA for patients with low OCT-1 activity (n = 47) was 0.367, compared with 0.635 (n = 46) for patients with high OCT-1 activity (P = .016) (Figure 5).
Dot plot showing the correlation between the OCT-1 activity and OCT-1 mRNA. Box plot demonstrating the difference between the levels of OCT-1 mRNA in the low and high OCT-1 activity groups.
Dot plot showing the correlation between the OCT-1 activity and OCT-1 mRNA. Box plot demonstrating the difference between the levels of OCT-1 mRNA in the low and high OCT-1 activity groups.
Interestingly, in the 77 patients where IC50 and OCT-1 mRNA analyses were available, there was no correlation (r = 0.162; P = .171) between the 2 parameters. Further grouping the patients into low and high IC50imatinib and assessing the level of OCT-1 mRNA expression revealed no significant difference between the 2 groups (low IC50imatinib n = 42, median 0.586; high IC50imatinib n = 35, median 0.546; P = .570). Limiting analysis to those patients where 24 months molecular follow-up is available (TIDEL patients), a significant difference in the molecular response between the 2 groups (low and high OCT-1 mRNA) could not be demonstrated (Table 3). Also, in contrast to OCT-1 activity, there was no significant difference between the 2 groups with respect to dose (600 mg or lower) and dose escalation (to 800 mg) (Table 3). Unlike OCT-1 activity, mRNA analysis did not reveal a group of patients with suboptimal response or at risk for imatinib failure (data not shown).
OCT-1 mRNA and molecular response
| Months after treatment . | Average molecular response at 6-month intervals . | ||
|---|---|---|---|
| 12 . | 18 . | 24 . | |
| mRNA | |||
| Low, n = 22 | 2.9 | 3.0 | 3.3 |
| High, n = 21 | 2.9 | 3.9 | 3.9 |
| P value | .693 | .09 | .125 |
| Low OCT-1 mRNA | |||
| Less than 600 mg, n = 10 | 2.8 | 2.9 | 2.9 |
| 600 mg or more, n = 12 | 2.8 | 3.1 | 3.3 |
| P value | .971 | .485 | .402 |
| High OCT-1 mRNA | |||
| Less than 600 mg, n = 8 | 2.5 | 3.6 | 3.7 |
| 600 mg or more, n = 13 | 2.9 | 3.9 | 3.9 |
| P value | .337 | .856 | .8 |
| Months after treatment . | Average molecular response at 6-month intervals . | ||
|---|---|---|---|
| 12 . | 18 . | 24 . | |
| mRNA | |||
| Low, n = 22 | 2.9 | 3.0 | 3.3 |
| High, n = 21 | 2.9 | 3.9 | 3.9 |
| P value | .693 | .09 | .125 |
| Low OCT-1 mRNA | |||
| Less than 600 mg, n = 10 | 2.8 | 2.9 | 2.9 |
| 600 mg or more, n = 12 | 2.8 | 3.1 | 3.3 |
| P value | .971 | .485 | .402 |
| High OCT-1 mRNA | |||
| Less than 600 mg, n = 8 | 2.5 | 3.6 | 3.7 |
| 600 mg or more, n = 13 | 2.9 | 3.9 | 3.9 |
| P value | .337 | .856 | .8 |
Data are log reductions except for P values.
BCR-ABL kinase domain mutations and OCT-1 activity
Five patients within our cohort of TIDEL patients developed BCR-ABL kinase domain mutations. Four of five patients had low OCT-1 activity. Three were removed from study due to progression prior to 12 months, the other being removed at 21 months. The one patient with high OCT-1 activity who developed a mutation remained on study for 30 months. Of the 5 patients, 3 had mutations within the P-loop (ATP binding domain of ABL), and 1 patient had multiple mutations.
Given that 4 of these 5 patients had low OCT-1 activity, the primary analysis of the predictive value of OCT-1 activity in molecular response was repeated after removing these patients from the analysis. This was to exclude the possibility that poor response in the low OCT-1 group was due to a higher risk of acquired resistance, rather than an inferior primary response to imatinib. There remained a significant difference between low (mean log-reduction in BCR-ABL of 3.0; n = 25) and high (mean log-reduction in BCR-ABL of 3.9; n = 27) OCT-1 activity groups in this modified dataset (P = .02 at 24 months). In addition, there remained a significant dose related difference within the low OCT-1 activity cohort (< 600 mg log reduction 2.5; > 600 log reduction 3.4; P = .019).
Patients with negligible OCT-1 activity
There were 5 patients who, in replicate assays, demonstrated IUR values without prazosin equal or lower than the values with prazosin (negligible; 0 ng/200 000 cells, OCT-1 activity) who all failed to achieve MMR by both 18 and 24 months. Three of five were intolerant of 600 mg imatinib, and all patients could not dose escalate at 12 months. Two of five patients had ABL kinase domain mutations (detected at 3 and 9 months, respectively). Three of five patients had low OCT-1 mRNA levels, 2 of these were the patients with BCR-ABL kinase mutations.
Discussion
The success of treatment with imatinib in the majority of chronic phase CML patients is well established. Improving treatment outcomes for those patients who perform less well, however, requires a detailed understanding of the critical determinants of treatment response. We and others have previously demonstrated that intrinsic sensitivity to imatinib-induced kinase inhibition is a good predictor of response,6,26 and that this is closely related to the intracellular uptake and retention (IUR) of imatinib.8 Further, it has been demonstrated that the active transport of imatinib is dependent on the organic cation transporter OCT-1.7,8 In this current study, examining a larger patient cohort, we confirm previous findings, that the IUR of imatinib correlates with the IC50imatinib, and that inhibition of OCT-1 with prazosin (α1-adrenoreceptor antagonist) removes the difference in IUR between the high- and low-IC50imatinib cohorts.
This current study sought to examine the functional activity of this protein and to explore the relationship between the amount of imatinib actively transported by OCT-1 and molecular response. The rationale for this approach was that OCT-1, like most drug transporters, is a complex protein, and while mRNA levels may be important,7,10 post-transcriptional regulation, protein level, and membrane localization12,27 are likely key determinants of functional activity. The combined effects of these variables can be most sensitively examined in an assay that provides a functional readout of activity.
We now demonstrate a significant correlation between the IC50imatinib and OCT-1 activity and show that patients with a low IC50imatinib have significantly greater OCT-1 activity than patients with high IC50imatinib. Comparing the OCT-1 activity with molecular response in all patients enrolled in TIDEL (chronic-phase newly diagnosed CML patients who received 600 mg imatinib up front), we demonstrate that patients with high OCT-1 activity achieved significantly greater molecular responses over 24 months of imatinib treatment than patients with low OCT-1 activity. While the specified dose was 600 mg in this patient cohort, because of tolerability issues, not all patients averaged 600 mg over the first 12 months. Separating patients into those averaging 600 mg and those failing to do so revealed patients with a high OCT-1 activity achieved good molecular responses regardless of dose. In contrast, the molecular response of those patients with low OCT-1 activity was highly dose dependent, with patients receiving 600 mg achieving significantly better molecular response by 24 months. These findings also held true when patients with BCR-ABL kinase domain mutations who ended study early were removed from analysis. This suggests that the OCT-1 activity is important in determining primary response to imatinib and that the significant difference observed between the 2 groups stratified on the basis of OCT-1 activity is not attributable to a small cohort of patients with secondary resistance. Furthermore, analysis of average daily dose received in the low- and high-OCT-1 activity cohorts reveals no significant difference (data not shown). This indicates that the significant difference in molecular response observed between groups is not simply a result of dose variation.
The TIDEL trial mandated dose increases if patients failed to reach various molecular milestones. In this cohort, 29 patients increased dose to 800 mg per day by 14 months, as a result of failure to achieve a 4-log reduction in BCR-ABL by 12 months. Assessing the effect of dose increase at 12 months and beyond revealed no significant effect in the high OCT-1 activity group. In contrast, patients with low OCT-1 activity whose dose increased achieved comparable molecular responses from 12 to 24 months as those in the high OCT-1 activity group, and this response was significantly superior compared with response in those patients who failed to dose increase. These data indicate that dose is a critical determinant of long-term molecular response in the low OCT-1 activity cohort, but patients with a high OCT-1 activity generally perform well regardless of dose.
Importantly, we also have identified a group of patients with low OCT-1 activity who were at higher risk for suboptimal or failed imatinib response if they did not receive the trial dose of 600 mg per day. The data presented here demonstrates clearly that dose is an important factor for overcoming suboptimal response in low OCT-1 activity patients. These findings indicate a potential role for increased imatinib dose up front in those patients found to have low OCT-1 activity prior to imatinib start. Intolerance to higher dose imatinib in these patients may be a trigger to switch to an ABL kinase inhibitor that is not dependent on OCT-1 for its uptake into cells. We have previously shown that nilotinib is not dependent on OCT-1 for its intracellular uptake.8 Other ABL kinase inhibitors are currently being tested.
One possible determinant of OCT-1 activity is the level of OCT-1 mRNA present. Using RQ-PCR we now demonstrate a correlation between the level of OCT-1 mRNA and OCT-1 activity and show that there is a significant difference in the level of mRNA between the low- and high-OCT-1 activity cohorts. Despite this correlation, it is of interest that the level of OCT-1 mRNA is not itself a strong predictor of molecular response in this de novo cohort, and the same sensitivity to dose is not demonstrable in the low OCT-1 mRNA cohort. These data suggest that while there is a correlation between OCT-1 activity and OCT-1 mRNA, the latter is not sufficient, as a single factor, to account for the observed differences between the 2 OCT-1 activity groups.
Other studies have demonstrated a significant link between OCT-1 mRNA levels and imatinib response.9,10 In the paper of Crossman,10 a significant difference was observed between mRNA levels in cytogenetic responders (CyR) and nonresponders (CyNR). Our current study differs from Crossman et al in that their study patients were assessed after an extensive period of prior therapy, whereas our patient cohort was comprised of previously untreated, de novo patients. Furthermore, in this current study 86% (49) of patients achieved CCyR by 12 months of therapy compared with 50% (15) (Crossman).
In the recent paper of Wang et al,9 70 patients were examined, but only 16 of these patients were previously untreated. High baseline OCT-1 mRNA was found to be a predictor for superior progression-free and overall survival at 5 years. While this study suggests that OCT-1 expression studies are of prognostic value in late chronic phase patients, this may not necessarily apply to de novo patients. Many of the patients studied in the Wang paper had developed resistance to interferon or had disease progression. These variables might impact OCT-1 mRNA expression.
In this current study 5 patients developed BCR-ABL kinase domain mutations. Four of these patients had low OCT-1 activity and were removed from study prematurely, in comparison to the one patient with high OCT-1 activity who remained on the drug for 30 months. While it is tempting to speculate about a link between OCT-1 activity and Abl kinase domain mutation development, particularly in the setting of dose sensitivity and superior molecular responses, the current data set remains too small.
Recent studies have suggested that the trough plasma level of imatinib may be a predictor of cytogenetic and molecular response. However, the critical determinant of the efficacy of imatinib therapy is the degree of kinase inhibition achieved, which is in turn determined by the intracellular concentration, rather than the plasma concentration, of imatinib. The intracellular concentration of imatinib is determined by the plasma concentration as well as the efficiency of uptake of the drug into the leukemic cells. The OCT-1 activity assay directly measures the efficiency of imatinib uptake. Whether a particular plasma imatinib level represents a satisfactory level or not may depend on the OCT-1 activity in that particular patient. Thus, a combination of plasma imatinib level and OCT-1 activity may allow individualized optimization of imatinib dose.
It is interesting to note that even under “optimal” circumstances—patients who averaged 600 mg of imatinib and had high OCT-1 activity—there were still approximately 20% who failed to achieve MMR by 18 months. We speculate that there are likely to be other causes for suboptimal response in these cases, perhaps not related to the adequacy of BCR-ABL kinase inhibition. However, we have demonstrated that lower-than-average doses of imatinib in patients with low OCT-1 activity account for the majority of cases of suboptimal response in this patient series.
In conclusion, differential function of the OCT-1 protein is a significant determinant of molecular response in chronic phase CML patients treated with imatinib. Importantly, using this functional assay to determine OCT-1 activity at the time of diagnosis may identify patients likely to respond well to standard-dose imatinib and those who would be most likely to benefit from a higher dose of imatinib.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the clinical consultants and trial coordinators involved in TOPS and TIDEL for the provision of patient samples. We thank Dr John Reynolds (ALLG Statistician) for provision of dose data and for critical review of the manuscript. We also acknowledge Dr Peter Diamond for his assistance with the OCT-1 mRNA assay.
This work was funded in part by grants from the Cancer Council of Australia and the National Health and Medical Research Council of Australia with support from Novartis Pharmaceuticals, Oceania, and Global.
Authorship
Contribution: D.W. designed and performed research, analyzed and interpreted data, performed statistical analyses, and wrote the manuscript. V.S., P.D., J.E., A.V., and S.Z. performed research. A.Z., K.L., and P.W.M. contributed to the manuscript preparation. T.H. designed research and contributed to the concept and design of the TIDEL study and contributed to the manuscript preparation.
Conflict-of-interest disclosure: K.L. and P.W.M. are both full-time employees of Novartis Pharmaceuticals. All other authors declare no competing financial interests.
Correspondence: Deborah White, Division of Haematology, IMVS & Hanson Institute, Frome Road Adelaide, Australia; e-mail: deb.white@imvs.sa.gov.au.