Abstract
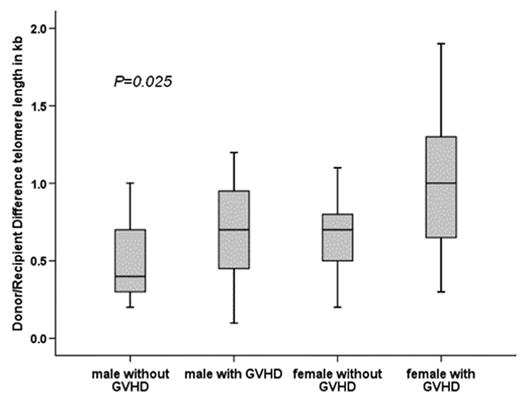
The establishment of donor-derived hematopoiesis in recipients of hematopoietic stem cell (HSC) transplantation (HSCT) involves extensive proliferation of HSCs and might lead to “premature aging” of hematopoietic cells. Telomere shortening as indicator of cell proliferation has been described after HSCT. The telomere attrition has mainly been observed during the first year after allogeneic HSCT. Thereafter, telomere length dynamics of recipients appeared not to differ from their donors. The aim of our cross-sectional study was to evaluate the telomere attrition in leukocyte subsets of very long term survivors (LTS) after HSCT in relation to donor/recipient age, sex, cell counts in peripheral blood, number of transplanted nucleated cells (NTNC), and occurrence of acute and chronic graft versus host disease (GVHD). Automated multicolour flow-FISH was used to measure the telomere length in granulocytes, naive T-cells, B-cells and NK/NKT-cells of 44 LTS and their HLA-identical sibling donors. At HSCT the median age of donors and recipients was 25.8 (2–46) and 26.8 years (5–50). The median follow-up after HSCT was 17.5 years (11–26). Four patients received HSCT for aplastic anemia, 40 for hematological malignancies. All patients received bone marrow as source of HSC and TBI was part of the conditioning in 39 (89%). Acute GVHD was observed in 31 (70%), and chronic GVHD in 22 (50%) patients. The age-matched and cell-type specific absolute telomere length values for recipients and donors fell between the 1st-99th percentile of the normal distribution. The telomere length (mean ± std) was significantly shorter in recipients as compared to their donors (p<0.01) for granulocytes (6.5±0.9 vs 7.1±0.9), T-cells (5.7±1.2 vs 6.6±1.2), B-cells (7.1±1.1 vs 7.8±1.1) and NK/NKT-cells (4.8±1.0 vs 5.6±1.3). The mean difference between recipients and donors for each subset of cells was between 0.6–0.9 kb. The mean telomere length of all subsets of cells was significantly shorter for males compared to females even though this difference was small. Age, sex of recipient, NTNC, and acute GVHD had no impact on telomere attrition. In all cell types except NK/NKT-cells we found a significant telomere shortening in recipients transplanted with a female donor (p<0.04) and in those with chronic GVHD (p<0.04). The telomere length difference between donor/recipient was most pronounced for recipients with the combination of a female donor and chronic GVHD (Figure 1). The loss in telomere length is more than twice for patients with a female donor and chronic GVHD compared to those with a male donor without chronic GVHD. In summary, our data reveal chronic GVHD and a female donor to be predictors for higher telomere attrition in LTS after HSCT. The more pronounced telomere attrition with a female donor and chronic GVHD corresponds to approximately 30–60 years of cell aging and raises once more the question of cellular immuno-senescence and its consequences in LTS of HSCT.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal