Abstract
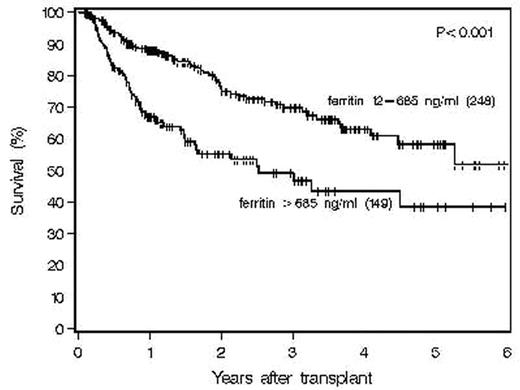
Iron overload is an adverse prognostic factor in patients who undergo allogeneic hematopoietic stem-cell transplantation (HSCT) for thalassemia and apparently in patients with acute leukemia and myelodysplastic syndrome as well. Iron overload has also been associated with susceptibility to infection following autotransplantation and with impaired immunity in other settings. We report here the results of a large study of consecutive patients undergoing autologous hematopoietic stem-cell transplantation (ASCT) for various hematologic malignancies to assess the influence of pretransplantation ferritin on outcomes following transplant. Pretransplantation ferritin, obtained within 100 days preceding transplant, was available on 397 patients undergoing autologous HSCT for various disorders (NHL=257, MM= 61, HD=58, AML=21) from 11/2/2000 to 12/28/2006.The median ferritin was 529.3 ng/ml (range, 12.8–4330). The median patient age was 52 (range, 19–77). Recursive partitioning analysis identified baseline ferritin >685 ng/ml as an adverse prognostic factor for survival as shown: ESTIMATED SURVIVAL BY PRETRANSPLANT FERRITIN LEVEL
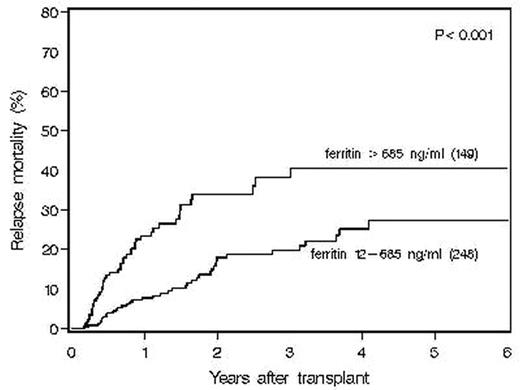
Age, gender, disease, disease status at transplant, interval from diagnosis to transplant, number of prior chemotherapy regimens, prior radiation therapy, ferritin, albumin, AST, Alkaline phosphatase, LDH, preparative regimen and CD 34+ dose were analyzed in a multivariable analysis. Elevated ferritin was an independent, adverse, significant prognostic factor for survival (p<0.001, HR=2.29),relapse-free survival (P<.001, HR=1.79) and relapse (p=0.006, HR=1.68). The addition of albumin, a negative acute phase reactant, did not change the prognostic impact of ferritin. Elevated ferritin was also significantly predictive of a higher incidence of relapse mortality (p<0.001) as shown below: RELAPSE MORTALITY BY FERRITIN LEVEL
Conclusions: Elevated ferritin adversely influences survival, relapse-free survival, and relapse mortality following autologous transplantation. Whether the increased number of deaths due to relapse is related to the established immunosuppressive affect of iron overload is unknown. Iron chelators have been shown to inhibit the growth of tumor cells in vitro and in vivo. Trials designed to analyze the benefit of iron chelation therapy prior to ASCT in patients with iron overload are warranted.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal