Abstract
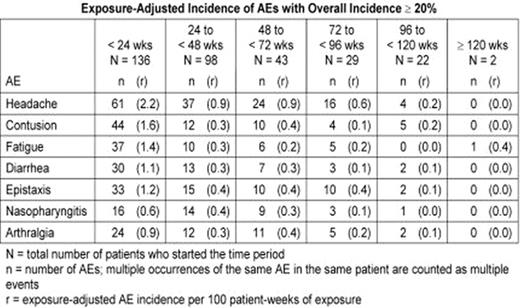
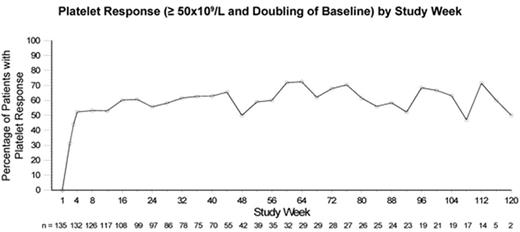
AMG 531, a novel thrombopoiesis-stimulating peptibody, increases platelet production by stimulating the TPO receptor. This study is an ongoing, open-label extension assessing the safety and efficacy of long-term, weekly, SC administration of AMG 531 in patients with chronic ITP who completed a previous AMG 531 study. Patients previously treated with AMG 531 received the same starting dose as the final dose given in the previous study; placebo-treated patients began the extension at 1μg/kg. Doses could be adjusted based on platelet response. As of an April 2007 analysis, 137 patients were enrolled and 136 had received AMG 531. The longest treatment duration was 122 weeks; 22 patients were followed for 96 weeks or longer. At baseline, 91 women and 46 men had a mean age of 53±15(SD) years and a median (range) platelet count of 18(1–50)x109/L; 82 patients (60%) had undergone splenectomy. The most frequently reported adverse events (AEs) were headache (overall incidence 31%), contusion (27%), fatigue (24%), diarrhea (24%), epistaxis (23%), nasopharyngitis (21%), and arthralgia (20%). Exposure-adjusted analysis showed no trend for AEs to increase in frequency with increased drug exposure (table). Eleven patients had serious AEs judged by investigators as treatment-related including 3 withdrawn from study (vaginal hemorrhage, increased reticulin in the bone marrow reported as myelofibrosis, and initial report of multiple myeloma later reclassified as monoclonal gammopathy of undetermined significance - 1 report each) and 8 who continued on-study (bone marrow disorder/reticulin fibrosis - 3, unacceptably high platelet count - 2, thrombosis - 2, and cerebral thrombosis/papilloedema/temporary blindness - 1). One patient developed neutralizing antibodies to AMG 531 (absent on retesting 4 months after drug cessation), with no clinical sequelae and no cross-reactive antibodies to TPO. Overall, 112 patients (82%) achieved a platelet response (≥50x109/L and doubling of baseline). From week 4 onward, weekly incidence of platelet response ranged from 52–73% (figure). Median number of weeks to first response was 2 (median dose 3μg/kg). Of 30 patients with baseline use of concurrent ITP therapies, 13 were able to discontinue them and 6 additional patients had a >25% dose reduction. Individualized weekly doses of AMG 531 provide a therapeutic option for long-term treatment of chronic ITP. The safety profile has been acceptable, and most patients have been able to maintain a platelet response and discontinue or reduce concurrent ITP therapies.
Platelet Response (≥ 50x109/L and Doubling of Baseline) by Study Week
Platelet Response (≥ 50x109/L and Doubling of Baseline) by Study Week
Author notes
Disclosure:Employment: L Bjorkquist, M Guo, J Nichol: Amgen. Consultancy: A Newland: GlaxoSmithKline, Protalex. Ownership Interests:; L Bjorkquist, M Guo, J Nichol: Amgen; JB Bussel: Amgen, GlaxoSmithKline. Research Funding: JB Bussel: Amgen, Biogen Idec, Cangene, Genentech, GlaxoSmithKline, Sysmex; A Newland: Amgen, GlaxoSmithKline; DJ Kuter, JThM de Wolf, TH Guthrie, JS Wasser, SI Hamburg: Amgen. Honoraria Information: A Newland: Amgen, Baxter, GlaxoSmithKline. Membership Information: JB Bussel: Amgen, Baxter, GlaxoSmithKline; A Newland: Amgen, Baxter, GlaxoSmithKline, Protalex.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal