Abstract
Background: In Japan, peripheral blood stem cell transplantation from an unrelated donor has not been approved. Therefore, for unrelated bone marrow transplantation with a reduced-intensity conditioning regimen (u-RIST), low-dose TBI has been added to facilitate engraftment. However, non-relapse mortality (NRM), which was mostly related to GVHD, was extremely high (54% at 1 year) after u-RIST with cladribine/busulfan/TBI 4 Gy (Kim et al. ASH 2006). To overcome this problem, we introduced antithymocyte globulin (Fresenius: ATG-F) at a lower dosage of 5–10 mg/kg to replace TBI. This study evaluated the feasibility of this regimen.
Patients and Methods :From January 2000 to May 2007, 65 patients with hematological malignancies received u-RIST with a conditioning regimen including fludarabine (Flu 30 mg/m2 x 6 days) or cladribine (2CdA 0.11 mg/kg x 6 days) plus busulfan (oral Bu 4 mg/kg x 2 days, iv Bu 3.2 mg/kg x 2 days) with 4 Gy TBI (n=30), 2 Gy TBI (n=20) or low-dose ATG-F (n=15). The median age of the patients was 57 years (range, 20–65). Their diagnosis included AML/MDS (n=39), lymphoma (n=19) and others (n=7). There were no differences in pretransplant disease status or HLA-disparity among the 3 different groups.
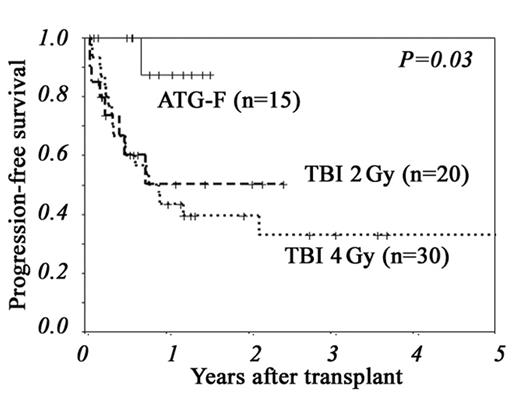
Results: The median follow-up of surviving patients was 381 days (range, 64–1832). Although more patients in the ATG-F group experienced graft failure, all 3 patients were rescued with a second transplant or DLI. Compared to low-dose TBI group, the incidences of grade II–IV and III–IV acute GVHD were significantly lower in the ATG-F group, which resulted in significantly lower NRM, better overall survival (OS) and better progression-free survival (PFS) (Figure). However, the incidences of disease relapse and CMV reactivation were not different among the 3 groups. A Cox proportional hazard model showed that low-dose ATG-F was associated with a significantly better PFS.
Conclusions: Our study showed that very low-dose ATG-F (5–10 mg/kg) significantly reduced the incidence of acute GVHD without an increase in the relapse rate, which led to a significantly improved PFS rate. A slightly higher rate of graft failure was manageable. The optimal dose of ATG-F needs to be determined according to the source of stem cells and HLA-disparities, including ethnic differences, and our study should help to provide a model to pursue this.
| . | TBI 4 Gy (n=30) . | TBI 2 Gy (n=20) . | LD ATG-F (n=15) . | P (TBI vs ATG-F) . |
|---|---|---|---|---|
| 2CdA/Flu | 11/19 | 5/15 | 0/15 | 0.01 |
| CSP/TAC | 28/2 | 4/16 | 4/11 | 0.01 |
| CR/non-CR, pretransplant | 10/20 | 9/11 | 5/10 | 0.74 |
| HLA match/mismatch | 17/13 | 13/7 | 10/5 | 0.64 |
| Graft failure | 3% | 0% | 20% | 0.04 |
| Acute GVHD, grade II–IV | 55% | 74% | 8% | <0.01 |
| Acute GVHD, grade III–IV | 31% | 16% | 0% | <0.01 |
| 1-year NRM | 46% | 15% | 0% | 0.01 |
| 1-year OS | 47% | 69% | 100% | <0.01 |
| 1-year Relapse | 19% | 40% | 12% | 0.43 |
| 1-year PFS | 43% | 51% | 88% | <0.01 |
| . | TBI 4 Gy (n=30) . | TBI 2 Gy (n=20) . | LD ATG-F (n=15) . | P (TBI vs ATG-F) . |
|---|---|---|---|---|
| 2CdA/Flu | 11/19 | 5/15 | 0/15 | 0.01 |
| CSP/TAC | 28/2 | 4/16 | 4/11 | 0.01 |
| CR/non-CR, pretransplant | 10/20 | 9/11 | 5/10 | 0.74 |
| HLA match/mismatch | 17/13 | 13/7 | 10/5 | 0.64 |
| Graft failure | 3% | 0% | 20% | 0.04 |
| Acute GVHD, grade II–IV | 55% | 74% | 8% | <0.01 |
| Acute GVHD, grade III–IV | 31% | 16% | 0% | <0.01 |
| 1-year NRM | 46% | 15% | 0% | 0.01 |
| 1-year OS | 47% | 69% | 100% | <0.01 |
| 1-year Relapse | 19% | 40% | 12% | 0.43 |
| 1-year PFS | 43% | 51% | 88% | <0.01 |
Figure
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal