Abstract
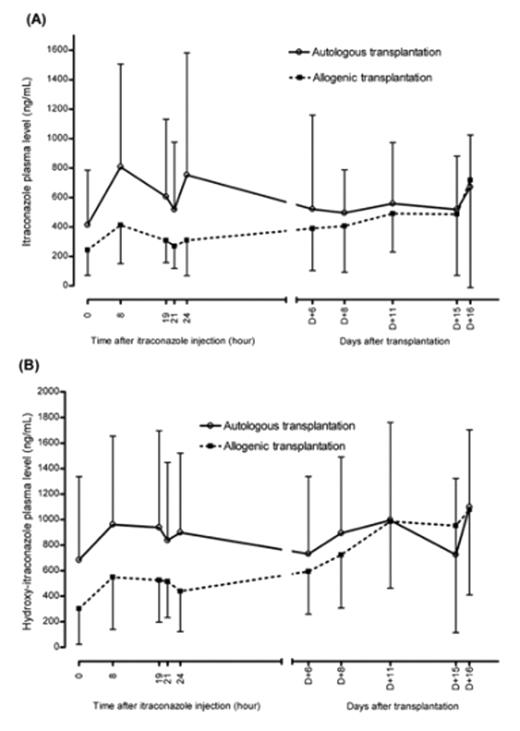
Invasive fungal infections are a major cause of morbidity and mortality for childhood patients who underwent stem cell transplantations. Patients who underwent SCT have varying degree of gastrointestinal complications. In this study, invasive fungal infection prophylaxis with intravenous itraconazole (ITR) administration to pediatric patients with SCT was performed in order to evaluate effectiveness and safety. Prospective examination was performed between jan. 2006 and Feb. 2007. Total 30 patients were enrolled. Patient group was divided into autologous and allogenic transplantation due to cyclosporin-A (CsA) use in allogenic transplantation courses. ITR (2.5mg/kg/dose) was administered twice daily from D+1 to D+2. After D+2, once daily administration of ITR (2.5mg/kg/dose) was performed until D+16. Each group constitutes with 15 patients. Sampling was performed after 48 hours after initial administration of ITR. Sampling times were as follows: D+3 (0hr), D+4 (8hr, 19hr, 21hr, 24hr) after infusion of ITR administration for drug level change monitoring, and trough level monitoring for ITR at D+6, D+8, D+11, D+15, D+16. Blood plasma ITR and hydroxyl-itraconazole (OH-ITR) were measured by HPLC technique. CsA level also measured concomittantly. Any evidences for fungal infection, liver and kidney dysfunction were monitored. Mean trough concentrations of ITR were greater than prophylactic level (250 ng/mL), except 1st time (D+3, 0hr) level as 243.39 ± 172.19 in allogenic transplant group (figure). ITR and OH-ITR level were highter in autologous than allogenic group at D+4, 8hr (figure). Trough levels of ITR and OH-ITR for both allogenic and autologous group were not different statistically (figure). Trough level of CsA was 189.9 ± 43.9 ng/mL. Average reduction rate of CsA was 69% (30%–100%, no case for increasing dose) from starting dose. Figure. Itraconazole (A) and hydroxy-itraconazole (B) plasma levels in pediatric stem cell transplantation recipients.
Figure
One case of mortality due to sepsis (not due to fungal infection) was reported in allogenic transplantation group. No case of proven fungal infection during study period. There are no renal toxicities greater than grade I. Five patients of autologous transplantation group had liver toxicity more than grade I but no more than grade III. Allogenic transplantation group patients showed only 2 patients with liver toxicity of grade I. Prophylactic intravenous ITR for childhood stem cell transplant recipients showed stable trough levels for both autologous and allogenic transplantation conditions with acceptable toxicities. After achievement of trough level, drug level change showed that 8 hour level after infusion of ITR (maintain once daily dose) revealed most high level. CsA level showed stable, but dose should reduce to 69% of original amount. CsA level should be carefully monotored in case of concomittant use of ITR.
Figure
One case of mortality due to sepsis (not due to fungal infection) was reported in allogenic transplantation group. No case of proven fungal infection during study period. There are no renal toxicities greater than grade I. Five patients of autologous transplantation group had liver toxicity more than grade I but no more than grade III. Allogenic transplantation group patients showed only 2 patients with liver toxicity of grade I. Prophylactic intravenous ITR for childhood stem cell transplant recipients showed stable trough levels for both autologous and allogenic transplantation conditions with acceptable toxicities. After achievement of trough level, drug level change showed that 8 hour level after infusion of ITR (maintain once daily dose) revealed most high level. CsA level showed stable, but dose should reduce to 69% of original amount. CsA level should be carefully monotored in case of concomittant use of ITR.
Author notes
Disclosure:Research Funding: This research was supported by Johnson & Johnson Korea pharmaceutical company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal