Abstract
Background: Many studies have evaluated the role of polyvalent immunoglobulins (IVIG) and CMV-hyperimmune IVIG (CMV-IVIG) prophylaxis in patients undergoing hematopoietic stem cell transplantation (HSCT), with inconsistent results and implications for practice.
Objectives: To evaluate the role of IVIG and CMV- IVIG prophylaxis in HSCT.
Methods: Systematic review and meta-analysis of randomized controlled trials comparing IVIG or CMV- IVIG with placebo or no intervention (control) for prophylaxis in HSCT. The Cochrane Library, MEDLINE, conference proceedings and references were searched until 2007. Primary outcome: all-cause-mortality ; Secondary outcomes: CMV infections, acute graft versus host disease (GVHD), interstitial pneumonitis (IP), veno-occlusive disease (VOD) and adverse events. Relative risks (RR) with 95% confidence intervals (CIs) were estimated and pooled.
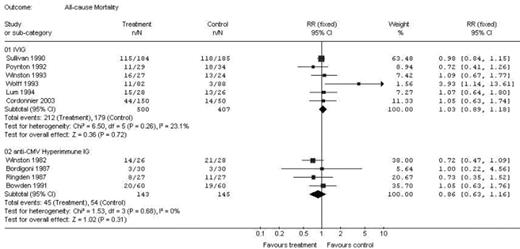
Results: Nineteen trials met inclusion criteria. IVIG was compared to control in 10 trials (7 IVIG in allogeneic HSCT, 2 allogeneic/autologous HSCT and 1 autologous HSCT). When IVIG was compared to control, there was no difference in all-cause mortality (RR 1.03; 95% CI 0.89–1.18, 6 trials, Fig.). There was a reduction in the number of CMV infections (RR 0.70; 95% CI 0.50–0.99, 4 trials) and in the number of episodes of IP (RR 0.61; 95% CI 0.43–0.87, 6 trials). There was no significant difference in the number of episodes of acute GVHD (RR 0.93; 95% CI 0.83–1.05, 6 trials). The risk for VOD was increased (RR 2.71; 95% CI 1.06–6.94, 3 trials) as were adverse events in the IVIG group (RR 8.12; 95% CI 3.15–20.9, 5 trials). CMV-IVIG was compared to control in 7 trials, all allogeneic HSCT. There was no difference in all-cause mortality (RR 0.86; 95% CI 0.63–1.16, 4 trials). In addition, there were no differences in the risk for developing CMV infections (RR 1.07; 95% CI 0.86–1.33, 7 trials), acute GVHD (RR 1.02; 95% CI 0.72–1.44, 5 trials) or IP (0.95; 95% CI 0.58–1.56, 5 trials).
Conclusions: Our review demonstrates that IVIG prophylaxis for HSCT reduces the risk for CMV infections and IP without a significant influence on mortality. The beneficial effects should be weighed against the higher incidence of adverse events, VOD, and cost associated with the use of prophylactic IVIG in HSCT patients. Conversely, the use of CMV-IVIG was not associated with a change in any of the parameters.
Outcomes Summary
| Outcome . | IVIG vs. control . | CMV-IVIG vs. control . |
|---|---|---|
| All Cause Mortality | RR 1.03; 95% CI 0.89–1.18 | RR 0.86; 95% CI 0.63–1.16 |
| CMV infections | RR 0.70; 95% CI 0.50–0.99 | RR 1.07; 95% CI 0.86–1.33 |
| acute GVHD | RR 0.93; 95% CI 0.83–1.05 | RR 1.02; 95% CI 0.72–1.44 |
| IP | RR 0.61; 95% CI 0.43–0.87 | RR 0.95; 95% CI 0.58–1.56 |
| VOD | RR 2.71; 95% CI 1.06–6.94 | No data |
| Adverse events | RR 8.12; 95% CI 3.15–20.9 | RR 7.0; 95% CI 0.38–129.34 |
| Outcome . | IVIG vs. control . | CMV-IVIG vs. control . |
|---|---|---|
| All Cause Mortality | RR 1.03; 95% CI 0.89–1.18 | RR 0.86; 95% CI 0.63–1.16 |
| CMV infections | RR 0.70; 95% CI 0.50–0.99 | RR 1.07; 95% CI 0.86–1.33 |
| acute GVHD | RR 0.93; 95% CI 0.83–1.05 | RR 1.02; 95% CI 0.72–1.44 |
| IP | RR 0.61; 95% CI 0.43–0.87 | RR 0.95; 95% CI 0.58–1.56 |
| VOD | RR 2.71; 95% CI 1.06–6.94 | No data |
| Adverse events | RR 8.12; 95% CI 3.15–20.9 | RR 7.0; 95% CI 0.38–129.34 |
Figure
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal