Abstract
Introduction: Prolonged treatment of graft vs. host disease (GVHD) is extremely immunosuppressive. Local therapy with intra-arterial (IA) injection of steroids may induce remission with lower extent of systemic immune suppression. Here, we present our experience with IA treatment of gastrointestinal (GI) and/or hepatic steroid resistant/dependent GVHD with 2 consecutive protocols.
Patients and methods: Thirty five patients (37 GVHD events (hepatic, n=15), (GI, n=16), (combined, n=6)) were treated with 53 IA sessions. Most side effects were minor.
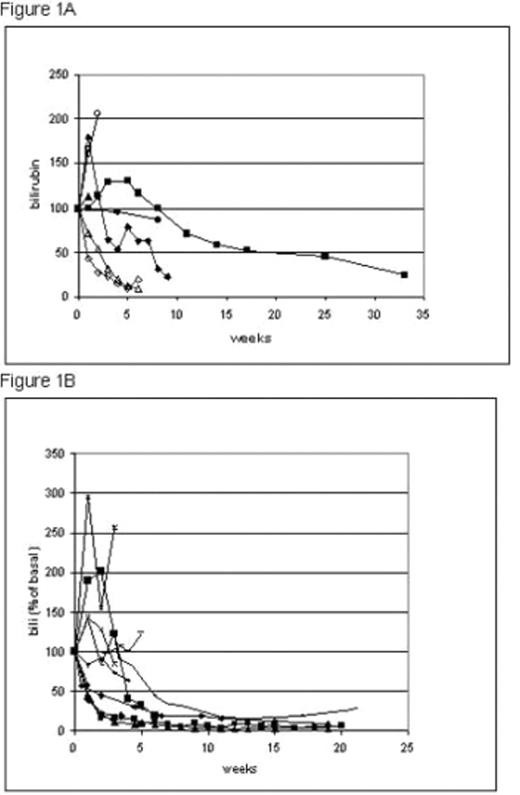
Results: We found that IA steroid therapy was associated with partial and complete remission among patients with steroid resistant/dependent hepatic or GI GVHD. Hepatic partial response was observed in 14 (66.6%) patients among whom 7 (33.3%) reached complete response. GI partial response was observed in 19 (86.4%) patients among whom 12 (54.4%) reached complete response. Early administration of the local therapy, female gender, myeloid basic disease, and a non-active status of the basic disease at the day of transplantation were found related for predicting a better response for the intra-arterial treatment. The use of high dose steroids in the hepatic IA protocol from was at least as good as intermediate dose steroids with methotrexate (table 1, figure 1) and may be safer.
Conclusions: Intra-arterial catheter guided steroid therapy is safe and effective in steroid resistant/dependent GVHD. Hepatic artery treatment with methotrexate can be safely substituted with high dose IA methylprednisolone. Further research is warranted characterizing the patients benefit most.
comparison between 1st and 2nd hepatic IA treatment protocols
| . | 1st protocol . | 2nd protocol . | Significance . |
|---|---|---|---|
| Median age (range) | 25 years (7–42) | 32 years (18–59) | P=0.09 |
| Sex (M:F) | 6:1 | 8:5 | NS |
| family donor vs MUD | 6:1 | 9:4 | NS |
| Median time in days SCT-GVHD (range) | 27 (13–133) | 45 (13–248) | NS |
| Median time in days GVHD-IA (range) | 15 (6–218) | 190 (12–2615) | P=0.09 |
| Median peak GVHD grade (range) | 3 (3–4) | 3 (2–4) | NS |
| Highest pre-IA treatment bilirubin level (in mmol/L; normal<17) (range) | 186 (138–321) | 225.5 (83–672) | NS |
| Median time to initial response in days (range) | 14.5 (4–100) | 8 (1–31) | P=0.073 |
| Median time to complete response in days (range) | 130.5 (35–226) | 49 (17–80) |
| . | 1st protocol . | 2nd protocol . | Significance . |
|---|---|---|---|
| Median age (range) | 25 years (7–42) | 32 years (18–59) | P=0.09 |
| Sex (M:F) | 6:1 | 8:5 | NS |
| family donor vs MUD | 6:1 | 9:4 | NS |
| Median time in days SCT-GVHD (range) | 27 (13–133) | 45 (13–248) | NS |
| Median time in days GVHD-IA (range) | 15 (6–218) | 190 (12–2615) | P=0.09 |
| Median peak GVHD grade (range) | 3 (3–4) | 3 (2–4) | NS |
| Highest pre-IA treatment bilirubin level (in mmol/L; normal<17) (range) | 186 (138–321) | 225.5 (83–672) | NS |
| Median time to initial response in days (range) | 14.5 (4–100) | 8 (1–31) | P=0.073 |
| Median time to complete response in days (range) | 130.5 (35–226) | 49 (17–80) |
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal