Abstract
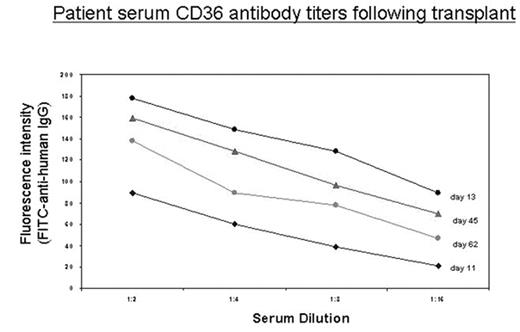
Platelet transfusion refractoriness following allogeneic bone marrow transplantation usually stems from HLA alloimmunization. However other platelet antigens may serve as targets of alloimmune reactivity. A 24-year-old Palestinian male with atypical CML in chronic phase underwent matched unrelated donor bone marrow transplant from a 39-year-old female, HLA-A, B, C and DR allele level matched donor. Conditioning was with busulfan 0.8mg/Kg IV q6 hours day -8 through -4, cyclophosphamide 60mg/Kg IV daily day -3 & -2 and rabbit anti-thymocyte globulin 1.5mg/Kg IV daily day -5 & -4. Graft versus host disease prophylaxis was with mini-dose methotrexate and tacrolimus, with filgrastim given for hematopoietic reconstitution. The cell dose was 1.86x106 CD34+ cells/Kg recipient body weight (3.66x108 mononuclear cells/Kg). Neutrophil engraftment occurred on day +14, and was complicated by a diffuse purpuric skin rash, with hypoxia and pulmonary infiltrates which resolved with corticosteroid therapy. His post-transplant course was also complicated by severe, transfusion refractory thrombocytopenia (to random donor pools, apheresis and crossmatched platelet units) starting on day +5 with platelet counts of <5x103/μL. He developed intra-retinal hemorrhage of the right eye, scleral hemorrhage bilaterally, epistaxis and muco-cutaneous bleeding. During the first 30 days following BMT he received 53 doses of platelets in addition to aminocaproic acid. He did not have DIC, splenomegaly or evidence of microangiopathy. HLA alloantibodies were not identified. Antigen Capture ELISA and flow cytometry for platelet specific antibody identification however demonstrated antibodies specific for CD36 in the patient’s serum (Fig). Platelets from the bone marrow donor typed positive for CD36. IVIG 0.5gm/Kg IV on d+13, 14, and 17 and Rituximab 375mg/m2 IV d + 17, 64, 72, and 82 were administered with no therapeutic response. The patient finally responded to transfusions of platelets collected from family members (parents, siblings and cousins) who were CD36negative. Bone marrow biopsies 2 months and 4 months following transplantation showed no evidence of residual disease with trilineage engraftment and adequate megakaryocytes, normal cytogenetics and >98% donor chimerism. As his immunosuppression has been tapered over time, the antibody titer has declined (Fig) as has his platelet transfusion requirement (41 doses transfused between day 31 and 120). However he remains dependent upon directed donor platelet transfusions from his CD36 negative family members at 120 days from transplant. The CD36 negative phenotype is rare in Caucasians, however, platelets from about 5% of individuals of Asian or African descent lack CD36. DNA sequencing to determine CD36 mutations in this patient is underway. We hypothesize that this patient developed a host vs. graft humoral response against donor derived and transfused platelets resulting in sustained severe thrombocytopenia.
Patient serum CD36 antibody titers following transplant
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal