Abstract
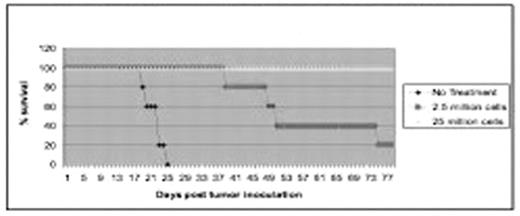
Medulloblastoma is the most common malignant brain tumor of childhood. Although the current overall survival has increased due to more aggressive therapy, the involved surgery, radiation and in some diseases high-dose chemotherapy have significant acute and long-term toxicities. T cell immunotherapy is a promising adjuvant for medulloblastoma because the immune response is specific for targeted antigens and is highly anatomically focused, occurring at the level of cell-to-cell interactions, thereby sparing adjacent normal brain tissue. Adoptive transfer of tumor-sensitized T cells has demonstrated therapeutic efficacy in animal tumor models, and clinical trials involving transfer of T lymphocytes are ongoing. Previous strategies have employed cultured human medulloblastoma cell lines as therapeutic targets, however priming of T cells using a spontaneously-arising medulloblastoma cell line in an immunocompetent mice model may be more representative of human cancer and more applicable to clinical practice. Spontaneous Murine medulloblastoma cell lines were created from knock-out Ptc+/−p53−/− mice and passaged in vivo. Brain tumors were established by transcranial inoculation of a single-cell suspension of murine medulloblastoma tumor cells in immunocompetent, C57BL/6N mice. Murine Medulloblastoma cell lines were rapidly tumorigenic and consistently fatal following intracranial inoculation in a dose-dependent manner. T-cells were obtained surgically from tumor draining lymph nodes (TDLN) in syngeneic mice and after initial purification, antigen-stimulated CD62Llow T cells were isolated by depletion of CD62Lhigh cells using MACS beads. T-cells were further activated ex vivo by incubation with anti-CD3 monoclonal antibody and exposure to media containing Interleukin-2 and Interleukin-7. Mice carrying 3-day established tumors receive systemic transfer of T cells by injection through the tail vein after 10 days of ex vivo culture. Mice typically receive sub-lethal total body irradiation (5 Gy) from a Cesium irradiator for lympho-depletion several hours before adoptive transfer. Mice were followed for survival and sacrificed when neurological symptoms were apparent as per Institutional Animal Care and Use Committee established guidelines. The activated T cells adoptively transferred to irradiated hosts with 3-day established intracranial MM1 tumors resulting in complete regression of established tumors (see figure). In addition, the above therapy demonstrated a dose-dependent effect, and the mice were cured without any evidence of neurological dysfunction caused by the anti-tumor response. This research suggests anti-medulloblastoma T cell vaccines may be promising for subsequent clinical testing.
Active Immunology of 3-day MMI medulloblastoma
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal