Abstract
Background: MCL is a prognostically unfavorable subtype of B-cell non-Hodgkin’s lymphoma, where autologous stem cell transplant (ASCT) is regarded a standard consolidation of the Ist-line therapy. However most of the pts are elderly or otherwise not illegible for intensive therapy protocols (i.e. only 6 out of 17 MCL patients diagnosed in our Dpt during last 6 months could be subjected to ASCT). Addition of Rituximab to induction chemotherapy increases the response rate, but still most patients subsequently relapse and some kind of consolidation is necessary.
Aims: It is postulated, that radioimmunotherapy with 90Y-Zevalin® (90Y-Ibritumomab tiuxetan), could be an efficient consolidation in patients not illegible for ASCT.
Methods: In our analysis we included 45 MCL cases subjected to RIT. The first 30 patients were enrolled in the Polish Lymphoma Research Group Trial (PLRG MCL1), a II phase multicenter study, where 90Y-Zevalin consolidated the response after FCM±R. The average follow-up of the study is now over 2 years (13 – 33 months). Further 15 cases with a shorter follow-up, initially treated with FC-R or CHOP - R, are included only in response and toxicity analysis. Only pts responding to initial chemotherapy (<25% BM infiltration, <30 mm lymph node and <12 cm spleen diameter), were offered Zevalin consolidation step. They received 2 doses of 250 mg/m2 R on days 1 and 8, followed by Z (0.3 or 0.4 mCi/Kg, based on initial PLT count; max dose=32 mCi). Responce rate and hematologic toxicity were determined. Response was monitored at 6 wks, 3 mo, and 3-mo intervals for up to 2 yrs to assess TTP.
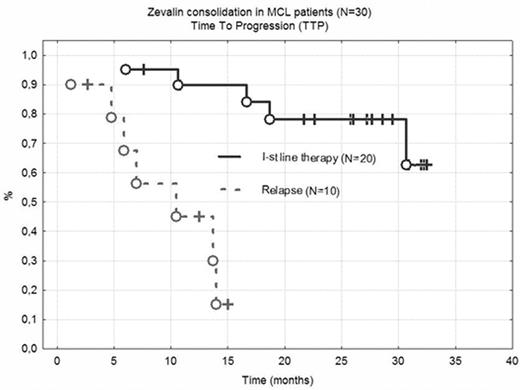
Results: 45 patients responding to initial chemotherapy (7 CR, 38 PR) were subjected to 90Y-Zevalin consolidation. A further regression was achieved in 37 patients (25 CR and 12 PR), one pt progressed. Hematological toxicity was important, with WBC < 2000/ul and Plt < 50 000/ul for 7.8 and 9.1 weeks respectively. In 3 cases (alive, in CR) cytopaenia was very prolonged (23, 24 and 40 weeks). One procedure related mortality was reported (hemorrhagic stroke at day +80). 32/45 pts required RBC or Platelet transfusions, 8/45 G-CSF support. No pts developed life-threatening infections, however hospital admission was necessary in 11 cases. Overall survival (OS) and time to progression (TTP) was analyzed in patients included in PLRG MCL1 trial. In 20 patients where RIT consolidated the 1st line therapy, at 30 months OS and TTP is 85% and 78% respectively (Fig 1). Responses are shorter in pts treated at relapse, even if CR is achieved after RIT (medium TTP and OS is 10 and 15 months).
Conclusions: Z consolidation is a feasible, outpatient approach for MCL patients not illegible for ASCT; it might have curative potential for patients treated at diagnosis or at first PR and may afford palliation for relapsed cases.
Zevalin consolidation in MCL patients (N=30) Time To Progression (TTP)
Zevalin consolidation in MCL patients (N=30) Time To Progression (TTP)
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal