Abstract
Background: The treatment of gastric mucosa-associated lymphoid tissue (MALT) lymphoma in the absence of H. pylori infection or when the lymphoma fails to regress after adequate antibiotic treatment remains controversial. Fludarabine (F) is an active agent for indolent lymphoma, however, its clinical activity in gastric MALT lymphoma has not been studied. The aim of the study is to assess the efficacy and safety of single-agent fludarabine in gastric MALT lymphoma and to analyze the molecular response (MR) after this treatment.
Methods: Treatment consisted of fludarabine (25 mg/m2 IV) given on days 1–5, every 4 weeks, for 6 cycles; after the first cycle, oral fludarabine was allowed to be given orally at 40 mg/m2 with the same schedule. Molecular response (MR) was assessed by RT-PCR analysis of t(11;18) or by PCR assays for analysis of IgH gene rearrangements analyzing FR1, FR2 and FR3 in endoscopic biopsies.
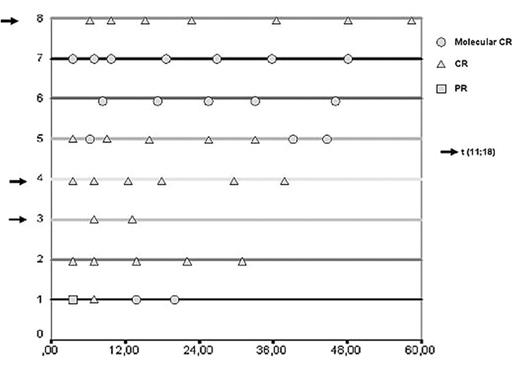
Results: Eight consecutive patients were included. Median age: 60 years (range: 45–77); 3 pts were in stage I, 2 stage II-1 and 3 stage IV according to Lugano system. Four out of 5 (80%) pts achieved a CR after three cycles and all eight cases (100%) achieved a CR after six cycles, for an overall response rate of 100%. After a median follow-up of 44 months (range 14.5–58 mo) no patient has shown clinical or endoscopic relapse. Hematological toxicity occurred in 75% of pts, mainly mild neutropenia and generally after the third cycle. Three cases received G-CSF (after the 2nd, 3rd and 6th cycle) and three patients required dose modification or delay (3–7 days) in the delivery of the following cycle. No blood transfusions were required. Only one patient had to be admitted because of non-neutropenic fever. None case of myelodisplasia has been detected at last follow-up. Four out of 8 pts (50%) achieved MR during the study-period (see figure). Four out of 5 (80%) pts without t(11;18) achieved MR. In contrast, no patient carrying t(11;18) achieved MR. Sequencing analysis of monoclonal PCR products will be presented.
Conclusions: Fludarabine, either intravenous or oral, is safe and achieve a high response rate when given in gastric MALT lymphoma, with many pts achieving MR. In those pts carrying t(11;18), residual disease can be detected by PCR but do not determine relapse at present follow-up.
Author notes
Disclosure:Research Funding: Ministerio de Sanidad y Consumo (PI03/0394). Off Label Use: This is a single center pilot study that follow local ethic regulations. All patients signed full informed consent. Despite the fact that fludarabine is not yet fully approved in MALT lymphoma, it is broadly used in all types of indolent lymphomas by the hematologic community (including marginal and MALT lymphomas). This study provides for the first time evidence regarding efficacy and toxicity of fludarabine in gastric MALT lymphoma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal