Abstract
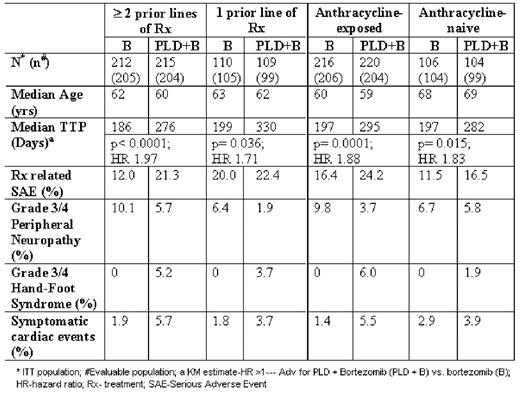
Recently, a significant improvement in time to progression (TTP) was reported for pegylated liposomal doxorubicin (PLD) + bortezomib combination therapy vs. bortezomib monotherapy in a phase III randomized trial in relapsed or refractory multiple myeloma (RRMM) (Orlowski, JCO 2007). This pre-specified analysis assessed the efficacy of PLD+bortezomib in RRMM based on the number of prior lines of therapy and previous anthracycline exposure status. Patients with ≥1 prior therapy were randomized to receive PLD at 30 mg/m2 on day 4 and bortezomib at 1.3 mg/m2 on days 1, 4, 8, and 11, or bortezomib alone for up to eight 21-day cycles, or at least 2 cycles beyond CR, or optimal response unless disease progression, or unacceptable toxicities occurred. The baseline beta-2 microglobulin levels were comparable between the subgroup with ≥2 prior treatments and that with 1 treatment (median 3.98 vs. 4.01 mg/L), as well as between anthracycline-exposed and -naïve patients (median 3.91 vs. 4.55 mg/L). The improved TTP reported previously with PLD+bortezomib over bortezomib in the total study population was similarly observed in all four subgroups of patients (patients with ≥2 lines of prior therapy or 1 prior therapy, anthracycline-exposed (median 144 mg/m2 in both treatment arms) or -naïve groups (Table)), indicating a consistent therapeutic benefit favoring the PLD+bortezomib combination. Furthermore, TTP was comparable for the PLD+bortezomib combination between groups with ≥2 lines of prior therapy vs. 1 prior therapy (heterogeneity test, p=0.523), as well as patients who were anthracycline-exposed or -naïve (heterogeneity test, p=0.716). Incidence of treatment-related SAEs, grade 3/4 neuropathy, and symptomatic cardiac events was comparable between treatment arms in each subgroup (Table). PLD-related hand-foot syndrome was also <10% in all PLD+bortezomib subgroups. These results demonstrate that PLD+bortezomib combination therapy significantly improves TTP compared to bortezomib alone, regardless of the number of prior lines of therapy, or anthracycline exposure.
Author notes
Disclosure:Employment: K. Hennessey, S. Mundle, and S. Zhuang are employees of Johnson & Johnson. Consultancy: J. Blade, Johnson & Johnson; J. San Miguel, Johnson & Johnson, Celgene, Pharmion; P. Sonneveld, Johnson & Johnson. Ownership Interests:; K. Hennessey, S. Mundle, and S. Zhuang own stock in Johnson & Johnson. Research Funding: J. Blade, Johnson & Johnson; T. Robak, Johnson & Johnson. Honoraria Information: J. Blade, Johnson & Johnson; J. San Miguel, Johnson & Johnson, Celgene, Pharmion; A. Spencer, Johnson & Johnson; JL Harousseau, Millennium. Membership Information: J. San Miguel, Johnson & Johnson, Celgene, Pharmion; A. Spencer, Johnson & Johnson; JL Harousseau, Johnson & Johnson; RZ Orlowski, Millennium and Johnson & Johnson.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal