Abstract
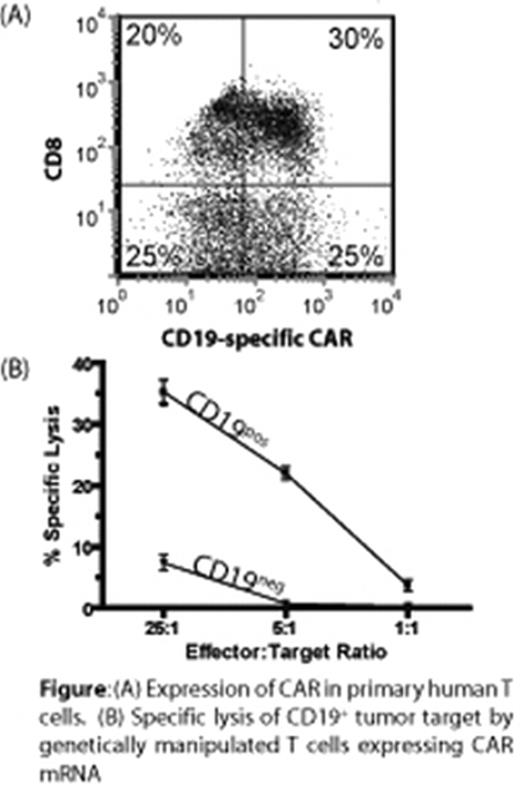
Pediatric B-lineage malignancies, such as acute lymphoblastic leukemia (B-ALL), generally have a poor prognosis upon relapse of tumor cells exhibiting resistance to conventional chemotherapy. In the absence of new commercially-available therapies for refractory pediatric cancers, it is up to pediatric oncologists to develop and apply new therapeutics. One candidate targeted therapy for B-lineage leukemia/lymphoma, is adoptive transfer of autologous T cells genetically modified to express a chimeric antigen receptor (CAR) and rendered specific for CD19. However, the broad adoption of T-cell gene therapy for children is limited by the expense to produce and release expression vectors and cell products and the risk of insertional mutagenesis. Therefore, we are developing an approach to express the CAR without integration of transgene and therefore without causing genotoxicity. Here we demonstrate that non-viral gene transfer of vector-free in vitro-transcribed messenger ribonucleic acid (mRNA) can efficiently introduce CAR into T cells. We demonstrate, using a commercially available electroporator, that codon-optimized mRNA coding for CD19-specific CAR is expressed on primary human T cells (Figure 1A) that redirects the specificity of CD19+ tumors (Figure 1B). When scaled-up for clinical trials, the electro-transfer of one or more mRNA species into T cells has the advantage of (i) ex vivo manipulation of T cells with preservation of homing receptors for lymph nodes and bone marrow (sites of leukemia/lymphoma), (ii) availability of clinically-meaningful numbers of genetically manipulated T cells within a day of apheresis, (iii) high levels of transgene expression, (iv) reduced cost (preparation of clinical-grade plasmid and mRNA compared with recombinant virus), and (iv) safety (no insertional mutagenesis). The relative ease of gene/mRNA transfer coupled with minimal manipulation of large numbers of T cells allows investigators to give patients multiple doses using existing infrastructure such as already widely exists for processing hematopoietic stem cells and blood banking. We are preparing a multi-institution clinical trial in which pediatric patients with refractory B-ALL receive personalized medication, prepared at each site, in the form of recursive infusions of CAR+ T cells repetitively electroporated with mRNA. This application of transient transfection to adoptive immunotherapy is expected to be of wide appeal for pediatric and adult patients alike with tumors that can be targeted by CAR.
(A) Expression of CAR in primary human T cells. (B) Specific lysis of CD19+ tumor target by genetically manipulated T cells expressing CAR mRNA
(A) Expression of CAR in primary human T cells. (B) Specific lysis of CD19+ tumor target by genetically manipulated T cells expressing CAR mRNA
Author notes
Disclosure:Research Funding: Federal, institutional, private and philanthropic funds were used in support of this project

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal