Abstract
We have recently demonstrated that Pax5 promotes B-lymphomagenesis by upregulating key components of B-cell receptor signaling [Cozma et al, J Clin Inv, 117 (8), 2007]. Gene regulation by Pax5 often involves complex formation with other oncogenic transcription factors of the Ets family, namely Myb and Ets1. We determined that expression of these proteins themselves depends on the presence of Pax5, as seen in human diffuse large B-cell lymphomas with Pax5 knockdown and murine lymphomas with epigenetic silencing of Pax5 [
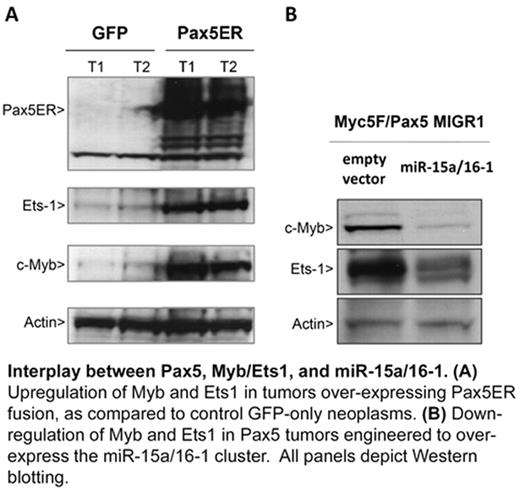
Interplay between Pax5, Myb/Ets1, and miR-15a/16-1. (A) Upregulation of Myb and Ets 1 in tumors over-expressing Pax5ER fusion, as compared to control GFP-only neoplasms. (B) Down-regulation of Myb and Ets1 in Pax5 tumors engineered to over-express the miR-15a/16-1 cluster. All panels depict Western blotting.
Interplay between Pax5, Myb/Ets1, and miR-15a/16-1. (A) Upregulation of Myb and Ets 1 in tumors over-expressing Pax5ER fusion, as compared to control GFP-only neoplasms. (B) Down-regulation of Myb and Ets1 in Pax5 tumors engineered to over-express the miR-15a/16-1 cluster. All panels depict Western blotting.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal