Abstract
Background: Peripheral T-cell lymphoma (PTCL) is a heterogeneous group of aggressive non-Hodgkin lymphomas (NHL) for which CHOP-type chemotherapy remains the standard despite its suboptimal results, especially when compared to its outcome in B-cell NHL. The International Peripheral T-Cell Lymphoma Clinical and Pathologic Review Project questioned the role of anthracyclines in the treatment of PTCL. To address this issue, we conducted a systematic literature review and meta-analysis of first-line therapy for untreated PTCL patients examining the complete remission (CR) and overall survival (OS) rates with anthracycline-based regimens. Given the established favorable treatment outcome of anaplastic large cell lymphomas (ALCL) along with the heterogeneity in response and survival rates across PTCL subgroups, we focused our analyses on non-ALCL PTCL and performed subgroup meta-analyses on the outcomes of anthracycline-based regimens for patients with PTCL- not-otherwise-specified (NOS), angio-immunoblastic T-cell lymphoma (AITL) and NK/T-cell NHL.
Methods: We searched the ASH and ASCO Annual Meeting Abstracts (2003–2006), MEDLINE (1/1996–6/2007), and Google Scholar. Each search used combinations of the term ’Peripheral T-Cell Lymphoma’, ’PTCL’, ’T Cell Lymphoma’, ’Non Hodgkin Lymphoma’, ’NK/T-cell lymphoma’, ’Angioimminoblastic lymphoma’, ’Anaplastic large cell lymphoma’, ’Enteropathy-type T-cell lymphoma’, ’Alk-negative’, ’Non-Alk positive’, ’Anthracycline’, ’Doxorubicin’, ’Adriamycin’, ’Intensive Chemo Therapy’, and ’CHOP’. Criteria for including studies were:
Intervention with chemotherapy with or without radiotherapy
Reporting in English of treatment outcome measures for patients with non-ALCL PTCL including CR rate, overall response (OR) rate, and at least one form of survival data.
Extracted data included pre-treatment disease status, treatment regimen, median follow up time, progression free survival, overall survival, CR, OR and early treatment-related death. Abstracts subsequently published as papers were excluded. In meta-analyses of selected studies, summary CR and 5-year OS estimates were calculated based on the assumption of fixed effects and using the Mantel-Haenszel method.
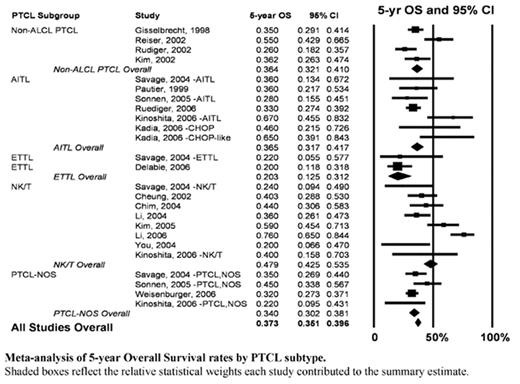
Results: Thirty-one studies meeting the inclusion criteria for this analysis were initially identified. These studies included data from 2912 patients. Twenty-five studies (n=2011) were evaluable for CR. Eighteen studies (n=1812) provided 5-year OS data. The estimated CR rate for anthracycline-based regimens among non-ALCL PTCL patients was 54.5% (95%CI 52.3%–56.8%), with subgroup CR rates as follows: AITL 54.7% (95%CI 47.3%–61.8%), NK/T 57.0% (95%CI 52.5%–61.5%), PTCL-NOS 55.6% (95%CI 51.8%–59.2%). The estimated 5-year OS for non-ALCL PTCL was 37.3% (95%CI 35.1%–39.6%), and for each subgroup was: AITL 36.5% (95%CI 31.7%–41.7%), NK/T 47.9% (95%CI 42.5%–53.5%), PTCL-NOS 34.0% (95%CI 30.2%–38.1%; Figure).
Conclusions: Despite the reasonable CR rates induced by anthracycline-based regimens in PTCL, OS remains poor. Future clinical trials need to focus on subtype-specific treatments for increasing CR and strategies such as stem cell transplantation or maintenance therapy, capable of sustaining CRs.
Meta-analysis of 5-year Overall Survival rates by PTCL subtype.
Shaded boxes reflect the relative statistical weights each study contributed to the summary estimate.
Meta-analysis of 5-year Overall Survival rates by PTCL subtype.
Shaded boxes reflect the relative statistical weights each study contributed to the summary estimate.
Author notes
Disclosure:Consultancy: Dr. Mary.Jo Lechowicz - Eisai Pharmaceuticals; Dr. Christopher Flowers - Genentech/Biogen/Idec. Research Funding: Dr. Christopher Flowers - Bayer, Johnson & Johnson, Millennium, Biovest. Membership Information: Dr. Christopher Flowers - Genentech/Biogen Idec

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal