Abstract
Background: We have previously reported results from a Phase 2 trial in which both treatment naïve (TN) and relapsed/refractory (R/R) pts with stable disease (SD) or a clinical response (PR or CR/CRu) following 4 weekly doses of rituximab received mitumprotimut-T active immunotherapy (
Treatment: Pts received rituximab (375mg/m2 iv weekly x 4) and those with ≥SD assessed at Week 11 received mitumprotimut-T, 1 mg sq every month (mo) x 6 starting on Week 12 along with Leukine (sargramostim, GM-CSF), 250 mcg, sq on Days 1–4. Pts continued to receive booster injections on a reduced schedule (every 2 mos x 6, then quarterly) until disease progression. Radiological scans were performed every 3 mos for the first 2 years then every 6 mos, and reviewed centrally. Objective response and progression were assessed using modified IWG criteria.
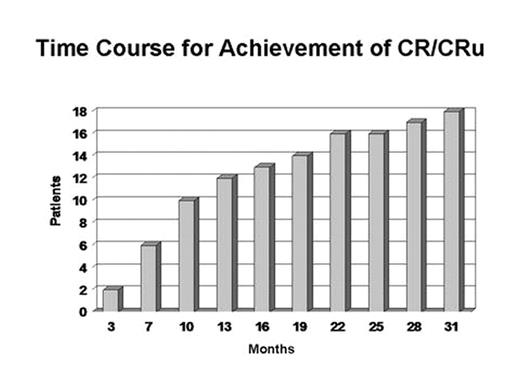
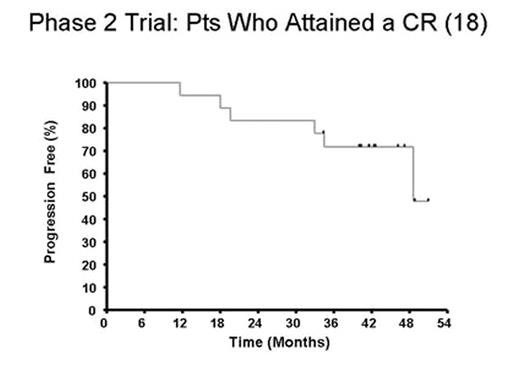
Results: 89 pts (54 RR, 35 TN) had ≥SD following rituximab received mitumprotimut-T + Leukine. Two pts (RR) achieved a CR following rituximab and prior to receiving mitumprotimut-T. As shown in Fig. 1, an additional 16 pts (9 TN, 7 RR) converted to CR/CRu over the next 31 mos. At a median follow-up of 42 mos, only 6 (3 TN, 3 RR) of the 18 CR pts have relapsed (Fig. 2).
Discussion: Long-term follow-up has shown a continuing conversion of pts into CR occurring as late as 31 mos. These data suggest clinical activity of mitumprotimut-T in these pts as determined by the increasing CR rate and the durability of this response. These data are of particular interest since a blinded interim analysis of best response in an ongoing controlled Phase 3 study of single agent rituximab vs. rituximab followed by mitumprotimut-T showed 47% of pts in this study to be in CR/CRu with 12–18 mos of follow-up (
Author notes
Disclosure:Employment: Richard Ghalie, M.D. is an employee of Favrille, Inc. Membership Information: Drs. Hainsworth, Kaplan, Holman, and Winter have all served as members of Favrille’s advisory committees. Off Label Use: Mitumprotimut-T is an investigational agent.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal