Abstract
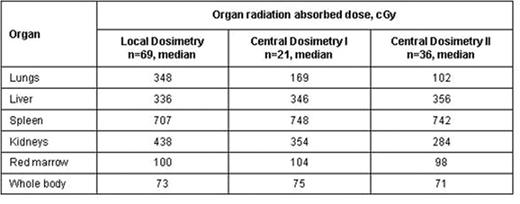
Yttrium-90 (90Y)-ibritumomab tiuxetan (Zevalin) is an anti-CD20 monoclonal antibody immunoconjugate linked to the pure beta-emitting 90Y radioisotope, which is known to allow targeted delivery limiting normal tissue exposure. As part of the international, phase 3 randomized First-line Indolent Trial (FIT) evaluating the efficacy of Zevalin consolidation compared with observation alone in patients (pts) with advanced stage follicular lymphoma achieving a response to first-line therapy, we investigated the radiation exposure of pts in the Zevalin arm for whom dosimetry data were available. Among the 414 pts enrolled in the trial (age ≥18 yrs, normal peripheral blood cell counts, <25% bone marrow involvement with lymphoma), 70 had a diagnostic scan with 111In-ibritumomab tiuxetan. Dosimetry was not completed in 1 pt. In addition to local dosimetry, central dosimetry was performed by a single experienced nuclear medicine physician in 57 of the 69 pts, including 21 pts in one participating hospital (I) and 36 evaluated in a central facility (II). All pts were injected with 185 MBq 111In-ibritumomab tiuxetan after infusion of rituximab (250 mg/m2) on day -7. At least 3 simultaneous anterior and posterior whole body (WB) scans were performed 15–45 min, 3–6 hrs, 1, 3–4 and 6 days after injection. Blood samples were drawn at corresponding intervals. Using the region of interest (ROI) technique, WB and organ (lungs, liver, spleen, kidneys) radioactivity was estimated from the geometric mean of anterior and posterior ROI counts. Whole blood aliquots were counted, normalized and decay corrected. WB, organ and red marrow (RM) radiation absorbed doses were calculated using the decay constant for 90Y and the projected activity (14.8 MBq/kg) with the MIRDOSE3 software.
Median dose in the 57 pts evaluated by central dosimetry was 102 cGy (range 28–327) to RM and 74 cGy (range 46–106) to WB. Radiation exposure was within the protocol-defined upper limits to normal organs (2000 cGy) in all pts and to RM (300 cGy) in all but 2 pts. In one pt, the RM dose was 85175 cGy by local dosimetry, but 94 cGy by central dosimetry; the pt was treated without complications. In the other pt, the RM dose was 327 cGy by central dosimetry, but 155 cGy by local. Average RM exposure was similar between pts with grade 3–4 neutropenia and/or thrombocytopenia and those with grade 0–2. A larger percentage (45%) of the 40 pts with grade 3 or 4 neutropenia had above-average RM radiation dose compared with 18% of the 17 pts with grade 0–2, whereas, the percentage of pts with above-average WB radiation dose was comparable in both groups (42% and 41%, respectively). Overall, neither WB nor RM radiation dose was correlated with hematologic toxicity (Spearman correlation). These findings are consistent with published data (
Author notes
Disclosure: Consultancy: A. BischofDelaloye - Bayer Schering Pharma and participation in Scientific Committee; C. Antonescu - Synaro; A. Hagenbeek - Roche Basel, Bayer Schering Pharma, and Genmab. Research Funding: A. BischofDelaloye - Participation in Multicenter Study. Honoraria Information: A. BischofDelaloye - Bayer Schering Pharma and Bayer (Schweiz) AG; C. Antonescu - Bayer Schering AG, A. Hagenbeek - Roche Basel, Bayer Schering Pharma, and Genmab. Off Label Use: Discussion of first-line consolidation with a product.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal