Abstract
Background: Although nearly all patients with idiopathic TTP will achieve remission, a majority of patients will suffer a recurrence at some point in the future. Our group has had an ongoing interest in using laboratory biomarkers to predict the risk of recurrence of idiopathic TTP after patients achieve remission and complete their initial therapy.
Method: Twenty patients (Table 1) with idiopathic TTP were analyzed beginning from the time of their initial presentation to our institution. 8 patients had a history of previous episodes of TTP. Patients were followed prospectively and monitored for relapse of TTP. Nine patients have maintained a continuous remission (Non-relapse) and 11 patients (Relapse) had a relapse of TTP. 139 serial samples from both groups, a median of 6.5/patient, were analyzed and compared in terms of ADAMTS13 activity, ADAMTS13 antibody concentration, and ADAMTS13 antigen. Median follow-up for the "Non-relapse" group was 39 months (range, 12 to 50). The median time to relapse was 11 months (range, 1.5 to 34) in the "Relapse" cohort. Data for the relapse group was censored at the time of relapse accounting for the shorter median follow-up of that cohort. The serial biomarkers from the "Relapse" group presented below reflect only samples obtained during continuous remission.
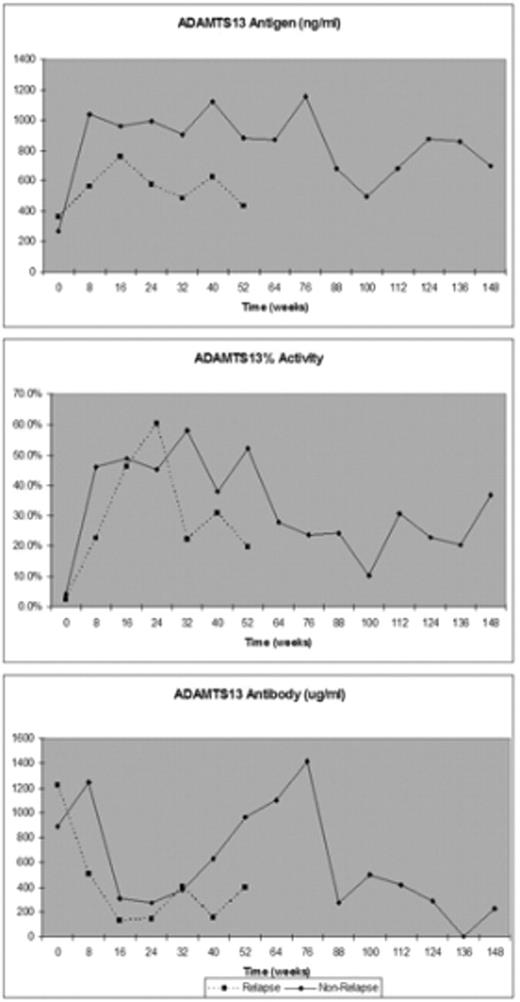
Results: Serial measurements of laboratory biomarkers are presented for both the "Relapse" and "Non-relapse" patients in Figure 1 below. During the first year of follow-up, the "Non-Relapse" patients had significantly higher ADAMTS13 Ag levels (p = 0.0065) compared to the patients who relapsed. There was no significant difference in the ADAMTS13 activity and antibody concentration between the two groups during the first year of follow up. In the 11 patients who relapsed, the mean ADAMTS13 activity was 1.2% (range, 0.5 – 3.2%), the mean antibody concentration was 958 ug/ml (range, 147 – 4,988), and the mean ADAMTS13 antigen was 221 ug/ml (range, 73 – 468) at the time of disease recurrence.
Conclusion: These data suggest that ADAMTS13 antigen determinations in the first year of follow-up may predict which patients will have a sustained remission of their disease. ADAMTS13 antigen measurement, compared to ADAMTS13 activity or antibody concentrations, may provide additional information to more accurately predict a patient’s future clinical course after achieving remission.
Demographic and Laboratory Data at Presentation
| . | . | . | . | Mean Laboratory Data at Presentation (range) . | |||
|---|---|---|---|---|---|---|---|
| . | N . | Age . | Sex . | Previous TTP . | ADAMTS13 % . | ADAMTS13 Ab(ug/ml) . | ADAMTS13 Ag. (ng/ml) . |
| Relapse | 11 | 37 (19–56) | 4M/7F | 4/11 | 2.5% (0.6 – 7.9) | 1,221 (190 – 3,397) | 360 (99 – 1,195) |
| Non-Relapse | 9 | 47 (21–59) | 2M/7F | 4/9 | 4.2% (<0.5 – 9.1) | 891 (59 – 4,040) | 266 (84 – 668) |
| . | . | . | . | Mean Laboratory Data at Presentation (range) . | |||
|---|---|---|---|---|---|---|---|
| . | N . | Age . | Sex . | Previous TTP . | ADAMTS13 % . | ADAMTS13 Ab(ug/ml) . | ADAMTS13 Ag. (ng/ml) . |
| Relapse | 11 | 37 (19–56) | 4M/7F | 4/11 | 2.5% (0.6 – 7.9) | 1,221 (190 – 3,397) | 360 (99 – 1,195) |
| Non-Relapse | 9 | 47 (21–59) | 2M/7F | 4/9 | 4.2% (<0.5 – 9.1) | 891 (59 – 4,040) | 266 (84 – 668) |
Figure
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal