Abstract
Background. The optimal duration of Oral Anticoagulant Therapy (OAT) for Deep Vein Thrombosis (DVT) can be tailored by Residual Vein Thrombosis (RVT) (
Objective of the study. In the present study, we evaluated the safety of withholding OAT, in patients with idiopathic DVT and without RVT, three months after the index thrombotic episode.
Study design. Prospective controlled study with two groups: patients without RVT stopped OAT after 3 months while those with RVT continued for additional 3 months.
Materials and Methods. Consecutive patients with a first episode of idiopathic DVT of the lower limbs; patients with cancer or known thrombophilia were excluded. At the third months of OAT, RVT was assessed as previously described; briefly, RVT was considered absent when a clot occupying less than 40% of the vein lumen was detected by compression ultrasonography. Events, classified as recurrent DVT and/or Pulmonary Embolism (PE) and/or major and minor bleeding were evaluated; all patients were followed-up for at least 12 months after OAT discontinuation.
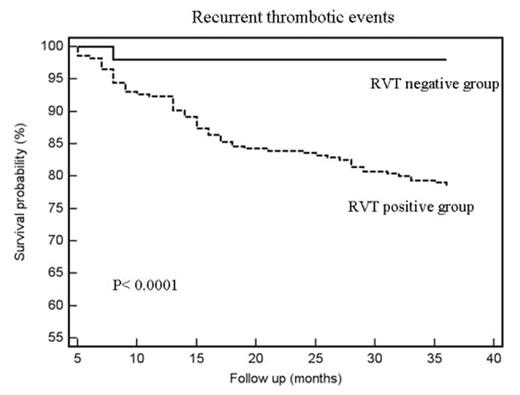
Results. During the period 1999–2006, 518 patients were included in the study. In 206 (39.7%) RVT was considered absent (RVT negative group) and they stopped OAT; the remaining 312 patients continued anticoagulants for additional 3 months (RVT positive group). Total duration of follow-up (FU) was 184.7 years for RVT negative group (with a mean FU of 3.0 ± 0.83 years) and 191.3 years for RVT positive group (with a mean FU of 3.1 ± 0.89 years). The rate and type of events during FU is reported in table and figure.
Conclusions. This investigation shows that in patients without RVT, three months of OAT are safe even after an episode of idiopathic DVT. This hold for at least 30% of the entire DVT population and has an important clinical impact; in fact, it is possible to select a group of patients with a very low risk for recurrences over a period of 3 years. This approach carries also a negligible risk for bleeding.
Events between RVT Negative and Positive Groups
| Outcomes . | RVT Neg. group (206) . | RVT Pos. group (312) . | "p" value . |
|---|---|---|---|
| *After OAT discontinuation, **During OAT | |||
| Recurrences, n/total (%)* | 2/206 (0.9) | 63/312 (20.2) | <0.0005 |
| Recurrences, n/100 person-year (%)* | 2/184.7 (1.1) | 63/191.3 (32.9) | <0.0005 |
| Type of recurrent VTE | |||
| DVT | 1 | 43 | |
| DVT + PE | 0 | 6 | |
| Isolated PE | 0 | 3 | |
| Controlateral | 1 | 11 | |
| Major bleeding, n/total (%)** | 0/206 | 3/312 (0.9) | |
| Major bleeding, n/100 person-Yr (%)** | 0/184.7 | 3/191.3 (1.5) | |
| Outcomes . | RVT Neg. group (206) . | RVT Pos. group (312) . | "p" value . |
|---|---|---|---|
| *After OAT discontinuation, **During OAT | |||
| Recurrences, n/total (%)* | 2/206 (0.9) | 63/312 (20.2) | <0.0005 |
| Recurrences, n/100 person-year (%)* | 2/184.7 (1.1) | 63/191.3 (32.9) | <0.0005 |
| Type of recurrent VTE | |||
| DVT | 1 | 43 | |
| DVT + PE | 0 | 6 | |
| Isolated PE | 0 | 3 | |
| Controlateral | 1 | 11 | |
| Major bleeding, n/total (%)** | 0/206 | 3/312 (0.9) | |
| Major bleeding, n/100 person-Yr (%)** | 0/184.7 | 3/191.3 (1.5) | |
Figure
Author notes
Disclosure: No relevant conflicts of interest to declare

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal