Abstract
Background: Low CD34+ cell mobilization in P.B. has been found in a quota of AML patients (10–30%). Contrary to what has been observed in CD34+ mobilization in other haematological afflictions, in AML no features pertaining to the disease or to the patient have been found to be predictive of CD34+ cell mobilization failure. A possible explanation for this particular aspect of CD34+ mobilization in AML patients could be an intrinsic abnormality of non leukemic hematopoietic cells determining an increased chemo-sensitivity to anti-neoplastic drugs. To test this hypothesis we assessed, in AML patients, frequency of various types of clonable precursors (CFUs) present in BM at the time of CR and their in vitro chemosensitivity. We also correlated this data with the efficiency of CD34+ cell mobilization in P.B.
Methods: 31 consecutive patients, affected with AML and a group of 15 normal BM donors were prospectively studied. Baseline CFU-GEMM, BFU-E, CFU-GM, CFU-E as well as the sensitivity of these precursors to two chemotherapeutic agents (ASTA-Z and VP-16) were assayed on BM cells obtained in first CR after consolidation chemotherapy. Chemo-sensitivity (100 - normalized residual CFU) was studied after short term in vitro incubation of bone marrow precursors at various drug concentrations. All pts underwent a CD34+ mobilization attempt and, as measure of mobilization strength, peaks of CD34+ cells reached in P.B. were determined.
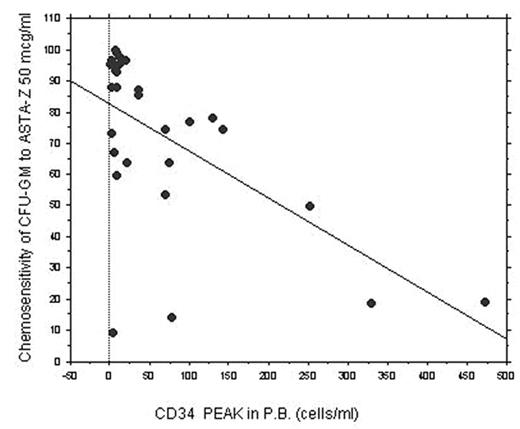
Results: In AML patients, after induction and consolidation schedules, a reduced number of all types of CFUs were found in BM compared to normal controls. The frequency of any types of CFUs and the chemo-sensitivity of CFU-GEMM, BFU-E and CFU-E were not correlated to CD34+ peak reached in P.B. However, in AML patients, an inverse correlation was found between chemo-sensitivity of CFU-GM and maximum CD34+ cells peak reached in P.B. during mobilization (r= − 0.807 and p=0.0001, when ASTA-Z was used at 100 mcg/ml). In univariate and multivariate logistic regression, chemo-sensitivity to ASTA-Z of CFU-GM was the only factor significantly associated with mobilization failure (P=0.02), independently of age and cytogenetical risk. Chemosensitivity of CFU-GEMM, BFU-E and CFU-E after in vitro incubation with chemotherapeutic drugs was not different in AML patients compared to CFU obtained from normal control. The contrary was found for CFU-GM and, overall, CFU-GM from AML patients had a significantly higher chemosensitivity to ASTA-Z compared to CFU-GM of normal controls (p= 0.01 at 50 mcg/ml).
Conclusions: We found that an abnormal high chemo-sensitivity of CFU-GM to some chemotherapy drugs in AML patients is associated with a high risk of CD34+ cell mobilization failure. This abnormality of non leukaemic bone marrow cells, present in CR, is restricted only to CFU-GM and is not evident in other CFUs.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal