Abstract
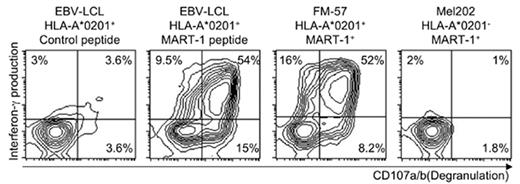
Immune responses to established cancer are difficult to evoke by vaccination of patients due to various mechanisms of tolerance. In contrast, T cells in allogeneic stem cell grafts can contribute to cure of a number of hematological malignancies by the graft-versus-leukemia (GvL) effect. However, unknown T cell specificities causing life-threatening graft-versus-host disease (GvHD) limit the applicability of this treatment. Selection of GvL activity would increase the efficiency, safety and applicability of allogeneic stem cell transplantation. Here, we describe a novel method for overcoming tolerance by generation of allo-restricted, antigen-specific T cells. These cells specifically recognize and kill melanoma tumor cells presenting the melanoma-associated MART-1 in context of the prevalent major histocompatibility complex (MHC) class I molecule HLA-A*0201. HLA-A*0201-negative peripheral blood mononuclear cells were co-cultured with autologous, monocyte-derived dendritic cells transfected with HLA-A*0201 mRNA and subsequently pulsed with the peptide ELAGIGILTV from the melanoma-associated antigen MART-1. Antigen-specific T cells were detected in 8/10 donors on day 12 using peptide-MHC-pentamers (median 0.28% of CD8+ T cells, range 0-4.17%), and in all donors by day 19. When needed, cells were restimulated on day 12 with HLA-A*0201 mRNA-transfected and peptide pulsed autologous EBV-transformed lymphoblastoid cell lines (EBV-LCL). MART-1 pentamer+ T cells from five donors were sorted and expanded. The cytotoxic T cell lines (CTLs) responded with production of interferon-γ, degranulation and lysis of target cells when stimulated with peptide-pulsed EBV-LCL, even at low peptide concentrations (10−5 M). Similar responses were seen to melanoma cell lines expressing HLA-A*0201 and MART-1 (FM-57, Malme3M). In contrast, MART-1+ melanoma cells lacking HLA-A*0201 (Mel202) induced responses only when transfected with HLA-A*0201. Starting out with 0.5×106 pentamer+ T cells, a potential number of 3.0×109 pentamer+ T cells could be reached four weeks later, representing a 7800-fold expansion Our data show that allo-restricted T cells with high specificity for tumor-associated antigens can be rapidly generated, sorted and expanded to clinically applicable numbers.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal