Abstract
Current treatment approaches for B-cell malignancies such as non-Hodgkin’s lymphoma and chronic lymphocytic leukemia are initially effective, but most patients ultimately relapse. Thus, there is a compelling need to develop novel therapies. One promising approach is immunotherapy using cytotoxic T cells (CTL). The major limitation of immunotherapy is the availability of large number of high-affinity autologous CTLs. Retargeting of T cells by genetic modification to introduce chimeric receptors can overcome this problem. Since infusion of permanently modified CTLs may not be desirable, we have developed a new method of transfecting CTLs using mRNA, which results in nearly universal but transient expression of a chimeric receptor targeting CD19. The chimeric immunoreceptor used for transfection was composed of anti-CD19 single chain antibody, CD3ζ and 4-1BB signaling domain (anti-CD19-CIR). mRNA was produced by in vitro transcription of PCR generated templates followed by poly(A) addition. CD8+ CTLs were isolated from expanded or resting T cells from healthy volunteers using magnetic beads. T cells were expanded using magnetic beads conjugated with anti-CD28 and anti-CD3. mRNA transfection was performed by electroporation using Amaxa nucleofector. The cytotoxicity was evaluated by a standard 51Cr release method. To test the in vivo function, CD19+ lymphoma cells expressing firefly luciferase (ffLuc) was injected to NOD/scid mice intraperitoneally. Intra peritoneal tumors have established by day 3, and anti-CD19-CIR or mock transfected T cells were injected intraperitoneally at days 3 and 6. The mice were imaged using IVIS biophotonic imaging system. When transfected with anti-CD19-CIR mRNA, more than 90% of the CD3+ T lymphocytes expressed the receptor on their surface. Both the CD4+ and the CD8+ subpopulations were transfected equally and the anti-CD19-CIR expression was sustained for a minimum of 3 days. T cells transfected with anti-CD19-CIR effectively lysed CD40L expanded autologous or allogeneic B cell lymphoblasts and multiple B-cell lymphoma cell lines (Daudi, SKI-DLCL-1 and CRL-2261). T cells expressing low, medium or high levels of anti-CD19-CIR effectively lysed the targets regardless of the level of expression. In vivo, anti-CD19-CIR transfected T cells were able to treat established tumors as demonstrated by non-invasive serial imaging of luciferase-mediated bioluminescence (Figure). Our data provide a rationale for developing methods for large-scale electroporation of chimeric receptors in CTLs, in support of clinical application of this treatment modality in patients with high-risk B-cell malignancies.
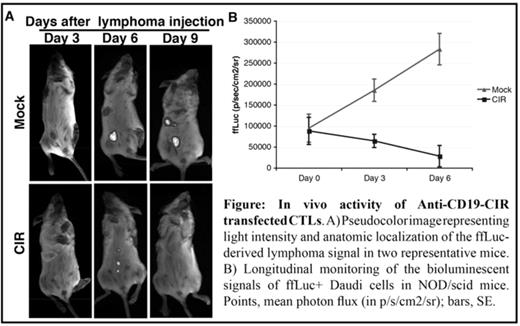
In vivo activity of Anti-CD19-CIR transfectedCTLs.A) Pseudocolorimage representing light intensity and anatomic localization of the ffLuc-derived lymphoma signal in two representative mice. B) Longitudinal monitoring of the bioluminescent signals of ffLuc+ Daudi cells in NOD/scid mice. Points, mean photon flux (in p/s/cm2/sr); bars, SE.
In vivo activity of Anti-CD19-CIR transfectedCTLs.A) Pseudocolorimage representing light intensity and anatomic localization of the ffLuc-derived lymphoma signal in two representative mice. B) Longitudinal monitoring of the bioluminescent signals of ffLuc+ Daudi cells in NOD/scid mice. Points, mean photon flux (in p/s/cm2/sr); bars, SE.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal