Abstract
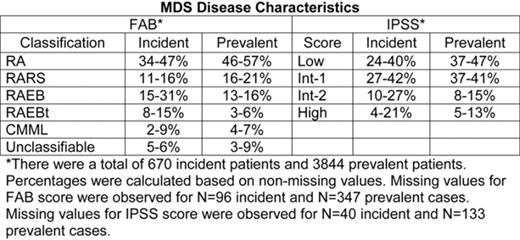
The estimated incidence of MDS in the United States is 3.6 per 100,000 people, or approximately 10,000 new cases per year. Few published studies about the characteristics of incident and prevalent cases exist. This study analyzes MDS patient (pt) demographics, classification, cytopenias, and treatment options as reported in surveys of AMA-listed hematology and/or medical oncology specialists. Physicians completed 6 distinct surveys on their 4 to 10 most recently seen MDS pts. Surveys were collected in June and Oct 2005; Jan, Apr, and Sept 2006; and Jan 2007. Data included information about demographics, disease information, hematologic status, transfusion dependency, and type and rationale for treatment given. Results are presented as minimum and maximum percentages observed across surveys. Per survey, 100 specialists were contacted; 77 to 97 physicians per survey returned information for at least one MDS pt, resulting in a total of 4528 pt surveys (621 to 827 per survey). Most (67–76%) had office-based practices; others were university, community, or VA hospital-based. Across surveys, 51-57% of pts were male. The median age was 72-74 years, with no marked difference between males and females. Median duration of MDS at the time of survey was 26-36 months. Secondary MDS was seen in 8-13% of pts, most following chemo (64-81%) or radiation therapy (10-22%). Lower-risk MDS was more common in follow-up (prevalent) MDS pts than in newly-diagnosed (incident) pts (Table). Cytogenetics were available on approximately 90% of all pts, with 68-71% classified (per IPSS) as low risk, 14-21% intermediate, and 11-16% poor. The most commonly reported abnormality was 5q- (7-9% of primary MDS cases). Platelet data were collected in the final 2 surveys: 37-42% of pts had counts <100,000mm3, and 15-18% <50,000mm3; the median transfusion trigger value was 15,000mm3. Low/Int-1 MDS pts were less dependent than Int-2/High-risk pts on both red blood cell transfusions (17–29% vs 52–67%) and platelet transfusions (3–10% vs 21–34%). Erythropoiesis stimulating agents (ESAs, such as erythropoietin or darbepoetin) were used by the majority of pts (68–74%), independent of IPSS risk. Most incident pts (57–65%) received supportive care with ESAs, and there was a trend towards increased use of non-ESA therapy over time (28% in 2005 to 40% in 2007). Among all pts, lenalidomide use was similar for Low/Int-1 vs Int-2/High-risk pts (0–12% vs 0–12%), as was use of azacitidine (41–53% vs 41–57%) and decitabine (12–60% vs 0-80%). Only a minority of MDS pts (1.5–4.2%) either had or were being considered for bone marrow transplantation. The proportion of MDS pts in clinical trials was also low (0.7–3.7%), and 14–18% of pts were categorized as watch and wait. In conclusion, the majority of MDS pts have lower-risk disease, and few have secondary MDS. Transfusion needs were greater, but ESA use similar, in higher-risk MDS compared to lower-risk pts. Transplantation, and clinical trial involvement, continues to be an option for only a minority of MDS pts.
Author notes
Disclosure:Employment: MS, JF: Amgen. Consultancy: MS: Celgene, Pharmion; AL: Celgene, Pharmion, Kanisa, SBIO; JM: Amgen. Ownership Interests:; JF: Amgen. Research Funding: MS: Celgene; AL: Celgene, Novartis. Honoraria Information: AL: Celgene, Pharmion, MGI Pharma. Membership Information: MS: Celgene, Pharmion; AL: Celgene, Pharmion, Kanisa, SBIO.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal